Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: A randomized controlled trial
- PMID: 34102020
- PMCID: PMC8242692
- DOI: 10.1002/sctm.21-0046
Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: A randomized controlled trial
Abstract
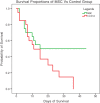
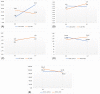
One of the main causes of acute respiratory distress syndrome in coronavirus disease 2019 (COVID-19) is cytokine storm, although the exact cause is still unknown. Umbilical cord mesenchymal stromal cells (UC-MSCs) influence proinflammatory T-helper 2 (Th2 ) cells to shift to an anti-inflammatory agent. To investigate efficacy of UC-MSC administration as adjuvant therapy in critically ill patients with COVID-19, we conducted a double-blind, multicentered, randomized controlled trial at four COVID-19 referral hospitals in Jakarta, Indonesia. This study included 40 randomly allocated critically ill patients with COVID-19; 20 patients received an intravenous infusion of 1 × 106 /kg body weight UC-MSCs in 100 ml saline (0.9%) solution (SS) and 20 patients received 100 ml 0.9% SS as the control group. All patients received standard therapy. The primary outcome was measured by survival rate and/or length of ventilator usage. The secondary outcome was measured by clinical and laboratory improvement, with serious adverse events. Our study showed the survival rate in the UC-MSCs group was 2.5 times higher than that in the control group (P = .047), which is 10 patients and 4 patients in the UC-MSCs and control groups, respectively. In patients with comorbidities, UC-MSC administration increased the survival rate by 4.5 times compared with controls. The length of stay in the intensive care unit and ventilator usage were not statistically significant, and no adverse events were reported. The application of infusion UC-MSCs significantly decreased interleukin 6 in the recovered patients (P = .023). Therefore, application of intravenous UC-MSCs as adjuvant treatment for critically ill patients with COVID-19 increases the survival rate by modulating the immune system toward an anti-inflammatory state.
Keywords: COVID-19; adjuvants; cord stem cell transplantation; cytokine release syndrome; immunology; mesenchymal stromal cells.
© 2021 The Authors. STEM CELLS TRANSLATIONAL MEDICINE published by Wiley Periodicals LLC on behalf of AlphaMed Press.
Conflict of interest statement
D.A. declared institutional funding from Ministry of Research, Technology and Higher Education, Indonesia. The other authors declared no potential conflicts of interest.
Figures
Similar articles
-
Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial.Stem Cells Transl Med. 2021 May;10(5):660-673. doi: 10.1002/sctm.20-0472. Epub 2021 Jan 5. Stem Cells Transl Med. 2021. PMID: 33400390 Free PMC article. Clinical Trial.
-
Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series.Stem Cell Res Ther. 2021 Jan 29;12(1):91. doi: 10.1186/s13287-021-02165-4. Stem Cell Res Ther. 2021. PMID: 33514427 Free PMC article. Clinical Trial.
-
Treatment of COVID-19-associated ARDS with mesenchymal stromal cells: a multicenter randomized double-blind trial.Crit Care. 2022 Feb 21;26(1):48. doi: 10.1186/s13054-022-03930-4. Crit Care. 2022. PMID: 35189925 Free PMC article. Clinical Trial.
-
Facing the Challenges in the COVID-19 Pandemic Era: From Standard Treatments to the Umbilical Cord-Derived Mesenchymal Stromal Cells as a New Therapeutic Strategy.Cells. 2023 Jun 19;12(12):1664. doi: 10.3390/cells12121664. Cells. 2023. PMID: 37371134 Free PMC article. Review.
-
Efficacy of umbilical cord mesenchymal stromal cells for COVID-19: A systematic review and meta-analysis.Front Immunol. 2022 Aug 29;13:923286. doi: 10.3389/fimmu.2022.923286. eCollection 2022. Front Immunol. 2022. PMID: 36105796 Free PMC article.
Cited by
-
Feasibility Study of Cord Tissue Derived Mesenchymal Stromal Cells in COVID-19-Related Acute Respiratory Distress Syndrome.Stem Cells Transl Med. 2023 Apr 17;12(4):185-193. doi: 10.1093/stcltm/szad009. Stem Cells Transl Med. 2023. PMID: 36929827 Free PMC article.
-
The safety and efficacy of mesenchymal stromal cells in ARDS: a meta-analysis of randomized controlled trials.Crit Care. 2023 Jan 20;27(1):31. doi: 10.1186/s13054-022-04287-4. Crit Care. 2023. PMID: 36670442 Free PMC article. Review.
-
Adipose-derived, autologous mesenchymal stem cell therapy for patients with post-COVID-19 syndrome: an intermediate-size expanded access program.Stem Cell Res Ther. 2023 Oct 5;14(1):287. doi: 10.1186/s13287-023-03522-1. Stem Cell Res Ther. 2023. PMID: 37798650 Free PMC article.
-
Bioengineering extracellular vesicle cargo for optimal therapeutic efficiency.Mol Ther Methods Clin Dev. 2024 Apr 26;32(2):101259. doi: 10.1016/j.omtm.2024.101259. eCollection 2024 Jun 13. Mol Ther Methods Clin Dev. 2024. PMID: 38770107 Free PMC article. Review.
-
Investigational Use of Mesenchymal Stem/Stromal Cells and Their Secretome as Add-On Therapy in Severe Respiratory Virus Infections: Challenges and Perspectives.Adv Ther. 2023 Jun;40(6):2626-2692. doi: 10.1007/s12325-023-02507-z. Epub 2023 Apr 17. Adv Ther. 2023. PMID: 37069355 Free PMC article. Review.
References
-
- Kaye RJ. Overview of stem cell therapy for acute respiratory distress syndrome with focus on COVID 19. Pain Physician. 2020;23(4S):421‐432. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

