XRCC1 prevents toxic PARP1 trapping during DNA base excision repair
- PMID: 34102106
- PMCID: PMC8294329
- DOI: 10.1016/j.molcel.2021.05.009
XRCC1 prevents toxic PARP1 trapping during DNA base excision repair
Abstract
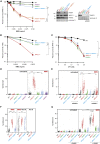
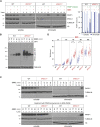
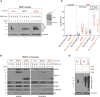
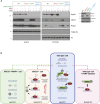
Mammalian DNA base excision repair (BER) is accelerated by poly(ADP-ribose) polymerases (PARPs) and the scaffold protein XRCC1. PARPs are sensors that detect single-strand break intermediates, but the critical role of XRCC1 during BER is unknown. Here, we show that protein complexes containing DNA polymerase β and DNA ligase III that are assembled by XRCC1 prevent excessive engagement and activity of PARP1 during BER. As a result, PARP1 becomes "trapped" on BER intermediates in XRCC1-deficient cells in a manner similar to that induced by PARP inhibitors, including in patient fibroblasts from XRCC1-mutated disease. This excessive PARP1 engagement and trapping renders BER intermediates inaccessible to enzymes such as DNA polymerase β and impedes their repair. Consequently, PARP1 deletion rescues BER and resistance to base damage in XRCC1-/- cells. These data reveal excessive PARP1 engagement during BER as a threat to genome integrity and identify XRCC1 as an "anti-trapper" that prevents toxic PARP1 activity.
Keywords: PARP inhibitors; PARP trapping; PARP1; XRCC1 protein complexes; base excision repair; single-strand breaks.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- Amé J.-C., Rolli V., Schreiber V., Niedergang C., Apiou F., Decker P., Muller S., Höger T., Ménissier-de Murcia J., de Murcia G. PARP-2, A novel mammalian DNA damage-dependent poly(ADP-ribose) polymerase. J. Biol. Chem. 1999;274:17860–17868. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

