Human Type II Taste Cells Express Angiotensin-Converting Enzyme 2 and Are Infected by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
- PMID: 34102107
- PMCID: PMC8179718
- DOI: 10.1016/j.ajpath.2021.05.010
Human Type II Taste Cells Express Angiotensin-Converting Enzyme 2 and Are Infected by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
Abstract
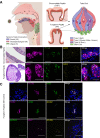
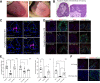
Chemosensory changes are well-reported symptoms of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. The virus targets cells for entry by binding of its spike protein to cell-surface angiotensin-converting enzyme 2 (ACE2). It is not known whether ACE2 is expressed on taste receptor cells (TRCs), or whether TRCs are infected directly. in situ hybridization probe and an antibody specific to ACE2 indicated presence of ACE2 on a subpopulation of TRCs (namely, type II cells in taste buds in taste papillae). Fungiform papillae of a SARS-CoV-2+ patient exhibiting symptoms of coronavirus disease 2019 (COVID-19), including taste changes, were biopsied. Presence of replicating SARS-CoV-2 in type II cells was verified by in situ hybridization. Therefore, taste type II cells provide a potential portal for viral entry that predicts vulnerabilities to SARS-CoV-2 in the oral cavity. The continuity and cell turnover of a patient's fungiform papillae taste stem cell layer were disrupted during infection and had not completely recovered 6 weeks after symptom onset. Another patient experiencing post-COVID-19 taste disturbances also had disrupted stem cells. These results demonstrate the possibility that novel and sudden taste changes, frequently reported in COVID-19, may be the result of direct infection of taste papillae by SARS-CoV-2. This may result in impaired taste receptor stem cell activity and suggest that further work is needed to understand the acute and postacute dynamics of viral kinetics in the human taste bud.
Keywords: ACE2; COVID-19; Taste; Type II Receptor Cell.
Published by Elsevier Inc.
Figures
References
-
- Lechien J.R., Chiesa-Estomba C.M., Beckers E., Mustin V., Ducarme M., Journe F., Marchant A., Jouffe L., Barillari M.R., Cammaroto G., Circiu M.P., Hans S., Saussez S. Prevalence and 6-month recovery of olfactory dysfunction: a multicentre study of 1363 COVID-19 patients. J Intern Med. 2021:451–461. - PubMed
-
- Menni C., Valdes A.M., Freidin M.B., Sudre C.H., Nguyen L.H., Drew D.A., Ganesh S., Varsavsky T., Cardoso M.J., El-Sayed Moustafa J.S., Visconti A., Hysi P., Bowyer R.C.E., Mangino M., Falchi M., Wolf J., Ourselin S., Chan A.T., Steves C.J., Spector T.D. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 2020;26:1037–1040. - PMC - PubMed
-
- Guerini-Rocco E., Taormina S.V., Vacirca D., Ranghiero A., Rappa A., Fumagalli C., Maffini F., Rampinelli C., Galetta D., Tagliabue M., Ansarin M., Barberis M. SARS-CoV-2 detection in formalin-fixed paraffin-embedded tissue specimens from surgical resection of tongue squamous cell carcinoma. J Clin Pathol. 2020;73:754–757. - PubMed
-
- Adamczyk K., Herman M., Fraczek J., Piec R., Szykula-Piec B., Zaczynski A., Wojtowicz R., Bojanowski K., Rusyan E., Krol Z., Wierzba W., Franek E. Sensitivity and specifity of prediction models based on gustatory disorders in diagnosing COVID-19 patients: a case-control study. medRxiv. 2020 [Preprint] doi: 10.1101/2020.05.31.20118380. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

