Enhancing myelin renewal reverses cognitive dysfunction in a murine model of Alzheimer's disease
- PMID: 34102111
- PMCID: PMC8298291
- DOI: 10.1016/j.neuron.2021.05.012
Enhancing myelin renewal reverses cognitive dysfunction in a murine model of Alzheimer's disease
Abstract
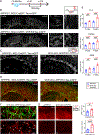
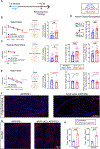
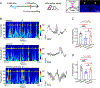
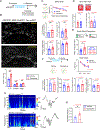
Severe cognitive decline is a hallmark of Alzheimer's disease (AD). In addition to gray matter loss, significant white matter pathology has been identified in AD patients. Here, we characterized the dynamics of myelin generation and loss in the APP/PS1 mouse model of AD. Unexpectedly, we observed a dramatic increase in the rate of new myelin formation in APP/PS1 mice, reminiscent of the robust oligodendroglial response to demyelination. Despite this increase, overall levels of myelination are decreased in the cortex and hippocampus of APP/PS1 mice and postmortem AD tissue. Genetically or pharmacologically enhancing myelin renewal, by oligodendroglial deletion of the muscarinic M1 receptor or systemic administration of the pro-myelinating drug clemastine, improved the performance of APP/PS1 mice in memory-related tasks and increased hippocampal sharp wave ripples. Taken together, these results demonstrate the potential of enhancing myelination as a therapeutic strategy to alleviate AD-related cognitive impairment.
Keywords: APP/PS1; SPW-R; clemastine; demyelination; myelination; oligodendroglia.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


Comment in
-
Myelin loss in AD.Nat Rev Neurosci. 2021 Aug;22(8):456-457. doi: 10.1038/s41583-021-00492-2. Nat Rev Neurosci. 2021. PMID: 34226716 No abstract available.
-
Replenishing our mind orchards: Enhancing myelin renewal to rescue cognition in Alzheimer's disease.Neuron. 2021 Jul 21;109(14):2204-2206. doi: 10.1016/j.neuron.2021.06.024. Neuron. 2021. PMID: 34293288
References
-
- Alzheimer A (1911). über eigenartige Krankheitsfälle des späteren Alters. Zeitschrift für die gesamte Neurologie und Psychiatrie 4, 356.
-
- Arranz AM, and De Strooper B (2019). The role of astroglia in Alzheimer’s disease: pathophysiology and clinical implications. Lancet Neurol 18, 406–414. - PubMed
-
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J. (2003) White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study. Archives of neurology 60: 393–398. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

