The tectum/superior colliculus as the vertebrate solution for spatial sensory integration and action
- PMID: 34102128
- PMCID: PMC8190998
- DOI: 10.1016/j.cub.2021.04.001
The tectum/superior colliculus as the vertebrate solution for spatial sensory integration and action
Abstract
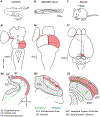
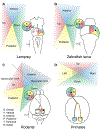
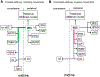
The superior colliculus, or tectum in the case of non-mammalian vertebrates, is a part of the brain that registers events in the surrounding space, often through vision and hearing, but also through electrosensation, infrared detection, and other sensory modalities in diverse vertebrate lineages. This information is used to form maps of the surrounding space and the positions of different salient stimuli in relation to the individual. The sensory maps are arranged in layers with visual input in the uppermost layer, other senses in deeper positions, and a spatially aligned motor map in the deepest layer. Here, we will review the organization and intrinsic function of the tectum/superior colliculus and the information that is processed within tectal circuits. We will also discuss tectal/superior colliculus outputs that are conveyed directly to downstream motor circuits or via the thalamus to cortical areas to control various aspects of behavior. The tectum/superior colliculus is evolutionarily conserved among all vertebrates, but tailored to the sensory specialties of each lineage, and its roles have shifted with the emergence of the cerebral cortex in mammals. We will illustrate both the conserved and divergent properties of the tectum/superior colliculus through vertebrate evolution by comparing tectal processing in lampreys belonging to the oldest group of extant vertebrates, larval zebrafish, rodents, and other vertebrates including primates.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


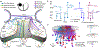

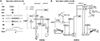
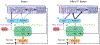
References
-
- Sparks DL (1986). Translation of sensory signals into commands for control of saccadic eye movements: role of primate superior colliculus. Physiol. Rev 66, 118–171. - PubMed
-
- Dean P, Redgrave P, and Westby GW (1989). Event or emergency? Two response systems in the mammalian superior colliculus. Trends Neurosci. 12, 137–147. - PubMed

