Transport mechanisms of plant hormones
- PMID: 34102450
- PMCID: PMC7615258
- DOI: 10.1016/j.pbi.2021.102055
Transport mechanisms of plant hormones
Abstract
Plant growth, development, and response to the environment are mediated by a group of small signaling molecules named hormones. Plants regulate hormone response pathways at multiple levels, including biosynthesis, metabolism, perception, and signaling. In addition, plants exhibit the unique ability to spatially control hormone distribution. In recent years, multiple transporters have been identified for most of the plant hormones. Here we present an updated snapshot of the known transporters for the hormones abscisic acid, auxin, brassinosteroid, cytokinin, ethylene, gibberellin, jasmonic acid, salicylic acid, and strigolactone. We also describe new findings regarding hormone movement and elaborate on hormone substrate specificity and possible genetic redundancy in hormone transport and distribution. Finally, we discuss subcellular, cell-to-cell, and long-distance hormone movement and local hormone sinks that trigger or prevent hormone-mediated responses.
Keywords: ABA; ABC transporters; Abscisic acid; Auxin; Brassinosteroid; Cytokinin; Ethylene; Gibberellin; Hormone transport; IAA; Jasmonic acid; NPF transporters; Phytohormones; Plant hormones; Salicylic acid; Strigolactone; Transport mechanisms.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
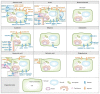
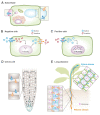
References
References and recommended reading
-
- Lacombe B, Achard P. Long-distance transport of phytohormones through the plant vascular system. Curr Opin Plant Biol. 2016;34:1–8. - PubMed
-
- Boursiac Y, Léran S, Corratgé-Faillie C, Gojon A, Krouk G, Lacombe B. ABA transport and transporters. Trends Plant Sci. 2013;18:325–333. - PubMed
-
- Santner A, Estelle M. Recent advances and emerging trends in plant hormone signalling. Nature. 2009;459:1071–1078. - PubMed
-
- Abualia R, Benkova E, Lacombe B. Transporters and Mechanisms of Hormone Transport in Arabidopsis. Adv Bot Res. 2018;87:115–138.
BioRxiv and unpublished
-
- Chen Jian, et al. ABCB-mediated auxin transport in outer root tissues regulates lateral root spacing in Arabidopsis. bioRxiv. 2020
-
- Vukašinović Nemanja, Wang Yaowei, Vanhoutte Isabelle, Fendrych Matyáš, Guo Boyu, Kvasnica Miroslav, Jiroutová Petra, Jana Oklestkova MSER. Local brassinosteroid biosynthesis enables optimal root growth Nemanja. bioRxiv. 2020:1–29. - PubMed
-
- Ackerman-Lavert M, Fridman Y, Matosevich R, Khandal H, Friedlander L, Vragović K, Ben El R, Horev G, Efroni I, Savaldi-Goldstein S. Auxin requirements for a meristematic state in roots depend on a dual brassinosteroid function. bioRxiv. 2020 - PubMed
Recommended reading
-
- Léran S, Noguero M, Corratgé-Faillie C, Boursiac Y, Brachet C, Lacombe B. Functional Characterization of the Arabidopsis Abscisic Acid Transporters NPF4.5 and NPF4.6 in Xenopus Oocytes. Front Plant Sci. 2020;11:1–6. [* This study tested ABA transport activity for several NPF4s family members in Xenopus oocyte system. The results show that the ABA transporter activities are pH-dependent and that there is no competitive inhibition of the ABA-analogs pyrabactin and quinabactin on ABA uptake] - PMC - PubMed
-
- Shohat H, Illouz-Eliaz N, Kanno Y, Seo M, Weiss D. The tomato della protein procera promotes abscisic acid responses in guard cells by upregulating an abscisic acid transporter. Plant Physiol. 2020;184:518–528. [** The authors discuss the negative crosstalk between gibberellin and ABA and demonstrate that PRO (a tomato DELLA protein), promotes guard cell responses. RNA-sequencing analysis of isolated guard cells revealed AIT1.1 as an ABA transporter in Solanum lycopersicum, which is being upregulated by PRO] - PMC - PubMed
-
- Pawela A, Banasiak J, Biała W, Martinoia E, Jasiński M. MtABCG20 is an ABA exporter influencing root morphology and seed germination of Medicago truncatula. Plant J. 2019;98:511–523. [*In this study, MtABCG20 is identified as an ABA exporter involved in seed germination and fine-tunes root morphology. It is proposed that MtABCG20 positively affects lateral root primordium formation and negatively affects the development of root nodulation in Medicago] - PMC - PubMed
-
- Qin P, Zhang G, Hu B, Wu J, Chen W, Ren Z, Liu Y, Xie J, Yuan H, Tu B, et al. Leaf-derived ABA regulates rice seed development via a transporter-mediated and temperature-sensitive mechanism. Sci Adv. 2021;7 [** In this study, DG1 was shown to control leaf-to-caryopsis ABA transport for regulating rice seed development. The DG1 dependant ABA translocation is temperature-sensitive as the process’s efficiency increases in high ambient temperature] - PMC - PubMed
-
- Skokan R, Medvecká E, Viaene T, Vosolsob S, Zwiewka M, Müller K, Skůpa P, Karady M, Zhang Y, Janacek DP, et al. PIN-driven auxin transport emerged early in streptophyte evolution. Nat Plants. 2019;5:1114–1119. [* The study elucidated the evolutionary origins of PINs in auxin mediate directional transport. The authors showed that the single PIN homologue of the green alga Klebsormidium flaccidum functions as a plasma membrane-localized auxin exporter in land plants and heterologous models] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

