PKM1 Exerts Critical Roles in Cardiac Remodeling Under Pressure Overload in the Heart
- PMID: 34102853
- PMCID: PMC8405569
- DOI: 10.1161/CIRCULATIONAHA.121.054885
PKM1 Exerts Critical Roles in Cardiac Remodeling Under Pressure Overload in the Heart
Abstract
Background: Metabolic remodeling precedes most alterations during cardiac hypertrophic growth under hemodynamic stress. The elevation of glucose utilization has been recognized as a hallmark of metabolic remodeling. However, its role in cardiac hypertrophic growth and heart failure in response to pressure overload remains to be fully illustrated. Here, we aimed to dissect the role of cardiac PKM1 (pyruvate kinase muscle isozyme 1) in glucose metabolic regulation and cardiac response under pressure overload.
Methods: Cardiac-specific deletion of PKM1 was achieved by crossing the floxed PKM1 mouse model with the cardiomyocyte-specific Cre transgenic mouse. PKM1 transgenic mice were generated under the control of tetracycline response elements, and cardiac-specific overexpression of PKM1 was induced by doxycycline administration in adult mice. Pressure overload was triggered by transverse aortic constriction. Primary neonatal rat ventricular myocytes were used to dissect molecular mechanisms. Moreover, metabolomics and nuclear magnetic resonance spectroscopy analyses were conducted to determine cardiac metabolic flux in response to pressure overload.
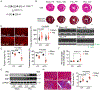
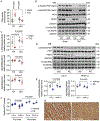
Results: We found that PKM1 expression is reduced in failing human and mouse hearts. It is important to note that cardiomyocyte-specific deletion of PKM1 exacerbates cardiac dysfunction and fibrosis in response to pressure overload. Inducible overexpression of PKM1 in cardiomyocytes protects the heart against transverse aortic constriction-induced cardiomyopathy and heart failure. At the mechanistic level, PKM1 is required for the augmentation of glycolytic flux, mitochondrial respiration, and ATP production under pressure overload. Furthermore, deficiency of PKM1 causes a defect in cardiomyocyte growth and a decrease in pyruvate dehydrogenase complex activity at both in vitro and in vivo levels.
Conclusions: These findings suggest that PKM1 plays an essential role in maintaining a homeostatic response in the heart under hemodynamic stress.
Keywords: cardiomegaly; glycolysis; heart failure; pyruvate dehydrogenase complex; pyruvate kinase.
Figures
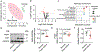





References
-
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al.Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. - PubMed
-
- Drazner MH. The progression of hypertensive heart disease. Circulation. 2011;123:327–334. - PubMed
-
- Nakamura M and Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018;15:387–407. - PubMed
-
- Hill JA and Olson EN. Cardiac plasticity. New Engl J Med. 2008;358:1370–1380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

