Epigenetic mechanisms underlying prostate cancer radioresistance
- PMID: 34103085
- PMCID: PMC8186094
- DOI: 10.1186/s13148-021-01111-8
Epigenetic mechanisms underlying prostate cancer radioresistance
Abstract
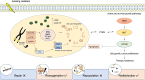
Radiotherapy (RT) is one of the mainstay treatments for prostate cancer (PCa), a highly prevalent neoplasm among males worldwide. About 30% of newly diagnosed PCa patients receive RT with a curative intent. However, biochemical relapse occurs in 20-40% of advanced PCa treated with RT either alone or in combination with adjuvant-hormonal therapy. Epigenetic alterations, frequently associated with molecular variations in PCa, contribute to the acquisition of a radioresistant phenotype. Increased DNA damage repair and cell cycle deregulation decreases radio-response in PCa patients. Moreover, the interplay between epigenome and cell growth pathways is extensively described in published literature. Importantly, as the clinical pattern of PCa ranges from an indolent tumor to an aggressive disease, discovering specific targetable epigenetic molecules able to overcome and predict PCa radioresistance is urgently needed. Currently, histone-deacetylase and DNA-methyltransferase inhibitors are the most studied classes of chromatin-modifying drugs (so-called 'epidrugs') within cancer radiosensitization context. Nonetheless, the lack of reliable validation trials is a foremost drawback. This review summarizes the major epigenetically induced changes in radioresistant-like PCa cells and describes recently reported targeted epigenetic therapies in pre-clinical and clinical settings.
Keywords: DNA repair; Epidrugs; Epigenetics; Prostate cancer; Radioresistance.
Conflict of interest statement
The authors have declared no conflict of interest.
Figures



References
-
- Morgan SC, Hoffman K, Loblaw DA, Buyyounouski MK, Patton C, Barocas D, et al. Hypofractionated radiation therapy for localized prostate cancer: executive summary of an ASTRO, ASCO, and AUA evidence-based guideline. Pract Radiat Oncol. 2018;8(6):354–360. doi: 10.1016/j.prro.2018.08.002. - DOI - PubMed
-
- Khuntia D, Reddy CA, Mahadevan A, Klein EA, Kupelian PA. Recurrence-free survival rates after external-beam radiotherapy for patients with clinical T1–T3 prostate carcinoma in the prostate-specific antigen era: what should we expect? Cancer. 2004;100(6):1283–1292. doi: 10.1002/cncr.20093. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
- AIRC-17217/Regione Campania
- iCURE-B21C17000030007/Regione Campania
- IDEAL:Identificazione e caratterizzazione di nuovi approcci terapeutici contro il cancro CUP B63D18000560007/Regione Campania
- MIUR, Proof of Concept-EPICUREPOC01_00043-B64I19000290008/Regione Campania
- P.O.R. CAMPANIA FSE 2014/2020 ASSE III-B27D18001070006/Regione Campania
LinkOut - more resources
Full Text Sources
Medical

