Senolytics reduce coronavirus-related mortality in old mice
- PMID: 34103349
- PMCID: PMC8607935
- DOI: 10.1126/science.abe4832
Senolytics reduce coronavirus-related mortality in old mice
Abstract
The COVID-19 pandemic has revealed the pronounced vulnerability of the elderly and chronically ill to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-induced morbidity and mortality. Cellular senescence contributes to inflammation, multiple chronic diseases, and age-related dysfunction, but effects on responses to viral infection are unclear. Here, we demonstrate that senescent cells (SnCs) become hyper-inflammatory in response to pathogen-associated molecular patterns (PAMPs), including SARS-CoV-2 spike protein-1, increasing expression of viral entry proteins and reducing antiviral gene expression in non-SnCs through a paracrine mechanism. Old mice acutely infected with pathogens that included a SARS-CoV-2-related mouse β-coronavirus experienced increased senescence and inflammation, with nearly 100% mortality. Targeting SnCs by using senolytic drugs before or after pathogen exposure significantly reduced mortality, cellular senescence, and inflammatory markers and increased antiviral antibodies. Thus, reducing the SnC burden in diseased or aged individuals should enhance resilience and reduce mortality after viral infection, including that of SARS-CoV-2.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures


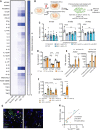
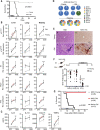

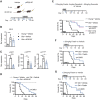
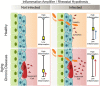
Comment in
-
Targeting aging cells improves survival.Science. 2021 Jul 16;373(6552):281-282. doi: 10.1126/science.abi4474. Science. 2021. PMID: 34437142 No abstract available.
References
-
- Palaiodimos L., Kokkinidis D. G., Li W., Karamanis D., Ognibene J., Arora S., Southern W. N., Mantzoros C. S., Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism 108, 154262 (2020). 10.1016/j.metabol.2020.154262 - DOI - PMC - PubMed
-
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506 (2020). 10.1016/S0140-6736(20)30183-5 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K99 AG058800/AG/NIA NIH HHS/United States
- K08 CA215105/CA/NCI NIH HHS/United States
- R37 AG013925/AG/NIA NIH HHS/United States
- P01 AG062413/AG/NIA NIH HHS/United States
- U24 AG056053/AG/NIA NIH HHS/United States
- R01 AG063543/AG/NIA NIH HHS/United States
- R01 AG013925/AG/NIA NIH HHS/United States
- R33 AG061456/AG/NIA NIH HHS/United States
- R00 AG058800/AG/NIA NIH HHS/United States
- R01 AG053832/AG/NIA NIH HHS/United States
- P30 CA077598/CA/NCI NIH HHS/United States
- P30 AG050886/AG/NIA NIH HHS/United States
- U19 AG056278/AG/NIA NIH HHS/United States
- R01 AI116678/AI/NIAID NIH HHS/United States
- P01 AG043376/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

