Hepatitis D virus in 2021: virology, immunology and new treatment approaches for a difficult-to-treat disease
- PMID: 34103404
- PMCID: PMC8355886
- DOI: 10.1136/gutjnl-2020-323888
Hepatitis D virus in 2021: virology, immunology and new treatment approaches for a difficult-to-treat disease
Abstract
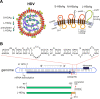
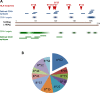
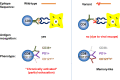
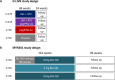
Approximately 5% of individuals infected with hepatitis B virus (HBV) are coinfected with hepatitis D virus (HDV). Chronic HBV/HDV coinfection is associated with an unfavourable outcome, with many patients developing liver cirrhosis, liver failure and eventually hepatocellular carcinoma within 5-10 years. The identification of the HBV/HDV receptor and the development of novel in vitro and animal infection models allowed a more detailed study of the HDV life cycle in recent years, facilitating the development of specific antiviral drugs. The characterisation of HDV-specific CD4+ and CD8+T cell epitopes in untreated and treated patients also permitted a more precise understanding of HDV immunobiology and possibly paves the way for immunotherapeutic strategies to support upcoming specific therapies targeting viral or host factors. Pegylated interferon-α has been used for treating HDV patients for the last 30 years with only limited sustained responses. Here we describe novel treatment options with regard to their mode of action and their clinical effectiveness. Of those, the entry-inhibitor bulevirtide (formerly known as myrcludex B) received conditional marketing authorisation in the European Union (EU) in 2020 (Hepcludex). One additional drug, the prenylation inhibitor lonafarnib, is currently under investigation in phase III clinical trials. Other treatment strategies aim at targeting hepatitis B surface antigen, including the nucleic acid polymer REP2139Ca. These recent advances in HDV virology, immunology and treatment are important steps to make HDV a less difficult-to-treat virus and will be discussed.
Keywords: antiviral therapy; chronic viral hepatitis; hepatitis D; immunology in hepatology.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SU: Advisory Board/Speaker Bureau for: GILEAD SCIENCES, MYR, VIRBIO, ASSEMBLY, JANSSEN, ENYO, PEPPERPRINT, ALIGOS. CN-H: Speaker Bureau for: ABBVIE, Falk Foundation, Novartis, MSD. PL: Advisory Board/Speaker Bureau for: BMS, ROCHE, GILEAD SCIENCES, GSK, ABBVIE, MSD, ARROWHEAD, ALNYLAM, JANSSEN, SBRING BANK, MYR, EIGER.
Figures