Stress granules, RNA-binding proteins and polyglutamine diseases: too much aggregation?
- PMID: 34103467
- PMCID: PMC8187637
- DOI: 10.1038/s41419-021-03873-8
Stress granules, RNA-binding proteins and polyglutamine diseases: too much aggregation?
Abstract
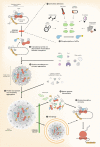
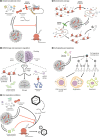
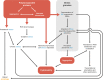
Stress granules (SGs) are membraneless cell compartments formed in response to different stress stimuli, wherein translation factors, mRNAs, RNA-binding proteins (RBPs) and other proteins coalesce together. SGs assembly is crucial for cell survival, since SGs are implicated in the regulation of translation, mRNA storage and stabilization and cell signalling, during stress. One defining feature of SGs is their dynamism, as they are quickly assembled upon stress and then rapidly dispersed after the stress source is no longer present. Recently, SGs dynamics, their components and their functions have begun to be studied in the context of human diseases. Interestingly, the regulated protein self-assembly that mediates SG formation contrasts with the pathological protein aggregation that is a feature of several neurodegenerative diseases. In particular, aberrant protein coalescence is a key feature of polyglutamine (PolyQ) diseases, a group of nine disorders that are caused by an abnormal expansion of PolyQ tract-bearing proteins, which increases the propensity of those proteins to aggregate. Available data concerning the abnormal properties of the mutant PolyQ disease-causing proteins and their involvement in stress response dysregulation strongly suggests an important role for SGs in the pathogenesis of PolyQ disorders. This review aims at discussing the evidence supporting the existence of a link between SGs functionality and PolyQ disorders, by focusing on the biology of SGs and on the way it can be altered in a PolyQ disease context.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Anderson P, Kedersha N. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Mol. Cell Biol. 2009;10:430–436. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

