Catalpol ameliorates depressive-like behaviors in CUMS mice via oxidative stress-mediated NLRP3 inflammasome and neuroinflammation
- PMID: 34103482
- PMCID: PMC8187638
- DOI: 10.1038/s41398-021-01468-7
Catalpol ameliorates depressive-like behaviors in CUMS mice via oxidative stress-mediated NLRP3 inflammasome and neuroinflammation
Abstract
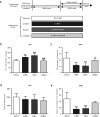
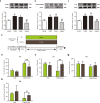
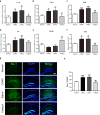
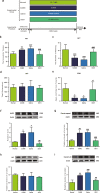
The purpose of the present study was to investigate whether catalpol exhibited neuroprotective effects in chronic unpredictable mild stress (CUMS) mice through oxidative stress-mediated nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin-domain-containing 3 (NLRP3) inflammasome and neuroinflammation. Deficits in behavioral tests, including open field test (OFT), forced swim test (FST), and elevated plus-maze test (EPM), were ameliorated following catalpol administration. To study the potential mechanism, western blots, quantitative real-time PCR (qRT-PCR) analysis and immunofluorescence imaging were performed on the hippocampus samples. We found that the defects of behavioral tests induced by CUMS could be reversed by the absence of NLRP3 and NLRP3 inflammasome might be involved in the antidepressant effects of catalpol on CUMS mice. Similar to the NLRP3 inflammasome, the expression of interleukin-1 beta (IL-1β), tumor necrosis factor alpha (TNF-α), and inducible nitride oxide synthase (iNOS) were increased after CUMS. The current study demonstrated that catalpol possessed anti-inflammatory effect on CUMS mice and inhibited microglial polarization to the M1 phenotype. In addition, the activity of mitochondrial oxidative stress might be involved in the NLRP3 activation, which was proved by the downregulation of NLRP3, apoptosis-associated speck-like protein containing a CARD (ASC), and cleaved IL-1β, after the administration of mitochondrion-targeted antioxidant peptide SS31. Taken together, we provided evidence that catalpol exhibited antidepressive effects on CUMS mice possibly via the oxidative stress-mediated regulation of NLRP3 and neuroinflammation.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Depressionhttp://www.who.int/mediacentre/factsheets/fs369/en/ (2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

