Synergistic immunotherapy of glioblastoma by dual targeting of IL-6 and CD40
- PMID: 34103524
- PMCID: PMC8187342
- DOI: 10.1038/s41467-021-23832-3
Synergistic immunotherapy of glioblastoma by dual targeting of IL-6 and CD40
Abstract
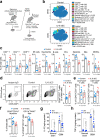
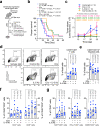
Immunologically-cold tumors including glioblastoma (GBM) are refractory to checkpoint blockade therapy, largely due to extensive infiltration of immunosuppressive macrophages (Mϕs). Consistent with a pro-tumor role of IL-6 in alternative Mϕs polarization, we here show that targeting IL-6 by genetic ablation or pharmacological inhibition moderately improves T-cell infiltration into GBM and enhances mouse survival; however, IL-6 inhibition does not synergize PD-1 and CTLA-4 checkpoint blockade. Interestingly, anti-IL-6 therapy reduces CD40 expression in GBM-associated Mϕs. We identify a Stat3/HIF-1α-mediated axis, through which IL-6 executes an anti-tumor role to induce CD40 expression in Mϕs. Combination of IL-6 inhibition with CD40 stimulation reverses Mϕ-mediated tumor immunosuppression, sensitizes tumors to checkpoint blockade, and extends animal survival in two syngeneic GBM models, particularly inducing complete regression of GL261 tumors after checkpoint blockade. Thus, antibody cocktail-based immunotherapy that combines checkpoint blockade with dual-targeting of IL-6 and CD40 may offer exciting opportunities for GBM and other solid tumors.
Conflict of interest statement
S.J.B. is an advisor to Bayer, Novocure, and Sumitomo Dainippon. S.J.B. has received research funding support from Incyte, GSK, Novocure, and Eli Lilly. L.Z. reports having received research funding from AstraZeneca, Bristol-Myers Squibb/Celgene, and Prelude Therapeutics. X.X. owns stocks in CureBiotech and Exio Bioscience. R.H.V. reports being an inventor on a licensed patent application for cellular immunotherapy. R.H.V. reports receiving royalties for the license of a research grade monoclonal antibody. Y.F. is a co-founder of Radix Therapeutics. The other authors have no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

