Novel Phenolic Compounds as Potential Dual EGFR and COX-2 Inhibitors: Design, Semisynthesis, in vitro Biological Evaluation and in silico Insights
- PMID: 34103896
- PMCID: PMC8178614
- DOI: 10.2147/DDDT.S310820
Novel Phenolic Compounds as Potential Dual EGFR and COX-2 Inhibitors: Design, Semisynthesis, in vitro Biological Evaluation and in silico Insights
Abstract
Introduction: Epidermal growth factor receptor (EGFR) inhibition is an imperative therapeutic approach targeting various types of cancer including colorectal, lung, breast, and pancreatic cancer types. Moreover, cyclooxygenase-2 (COX-2) is frequently overexpressed in different types of cancers and has a role in the promotion of malignancy, apoptosis inhibition, and metastasis of tumor cells. Combination therapy has been emerged to improve the therapeutic benefit against cancer and curb intrinsic and acquired resistance.
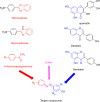
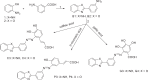
Methods: Three semi-synthetic series of compounds (C1-4, P1-4, and G1-4) were prepared and evaluated biologically as potential dual epidermal growth factor receptor (EGFR) and COX-2 inhibitors. The main phenolic constituents of Amaranthus spinosus L. (p-coumaric, caffeic and gallic) acids have been isolated and subsequently subjected to diazo coupling with various amines to get novel three chemical scaffolds with potential anticancer activities.
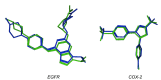
Results: Compounds C4 and G4 showed superior inhibitory activity against EGFR (IC50: 0.9 and 0.5 µM, respectively) and displayed good COX-2 inhibition (IC50: 4.35 and 2.47 µM, respectively). Moreover, the final compounds were further evaluated for their cytotoxic activity against human colon cancer (HT-29), pancreatic cancer (PaCa-2), human malignant melanoma (A375), lung cancer (H-460), and pancreatic ductal cancer (Panc-1) cell lines. Interestingly, compounds C4 and G4 exhibited the highest cytotoxic activity with average IC50 values of 1.5 µM and 2.8 µM against H-460 and Panc-1, respectively. The virtual docking study was conducted to gain proper understandings of the plausible-binding modes of target compounds within EGFR and COX-2 binding sites.
Discussion: The NMR of prepared compounds showed characteristic peaks that confirmed the structure of the target compounds. The synthesized benzoxazolyl scaffold containing compounds showed inhibitory activities for both COXs and EGFR which are consistent with the virtual docking study.
Keywords: BRAF; anti-inflammatory; anticancer; kinase inhibitors; multitarget agents.
© 2021 Abdelgawad et al.
Conflict of interest statement
The authors reported no conflicts of interest for this work.
Figures



Similar articles
-
EGFR and COX-2 Dual Inhibitor: The Design, Synthesis, and Biological Evaluation of Novel Chalcones.Molecules. 2022 Feb 9;27(4):1158. doi: 10.3390/molecules27041158. Molecules. 2022. PMID: 35208952 Free PMC article.
-
New oxadiazoles with selective-COX-2 and EGFR dual inhibitory activity: Design, synthesis, cytotoxicity evaluation and in silico studies.Eur J Med Chem. 2019 Dec 1;183:111693. doi: 10.1016/j.ejmech.2019.111693. Epub 2019 Sep 10. Eur J Med Chem. 2019. PMID: 31539778
-
Optimization of pyrazole/1,2,4-triazole as dual EGFR/COX-2 inhibitors: Design, synthesis, anticancer potential, apoptosis induction and cell cycle analysis.Eur J Med Chem. 2025 Apr 5;287:117340. doi: 10.1016/j.ejmech.2025.117340. Epub 2025 Jan 30. Eur J Med Chem. 2025. PMID: 39914141
-
Novel benzothiazole-based dual VEGFR-2/EGFR inhibitors targeting breast and liver cancers: Synthesis, cytotoxic activity, QSAR and molecular docking studies.Bioorg Med Chem Lett. 2022 Feb 15;58:128529. doi: 10.1016/j.bmcl.2022.128529. Epub 2022 Jan 7. Bioorg Med Chem Lett. 2022. PMID: 35007724 Review.
-
2-Anilinopyrimidine derivatives: Design, synthesis, in vitro anti-proliferative activity, EGFR and ARO inhibitory activity, cell cycle analysis and molecular docking study.Bioorg Chem. 2020 Jun;99:103798. doi: 10.1016/j.bioorg.2020.103798. Epub 2020 Mar 29. Bioorg Chem. 2020. PMID: 32247112 Review.
Cited by
-
The Benzoxazole Heterocycle: A Comprehensive Review of the Most Recent Medicinal Chemistry Developments of Antiproliferative, Brain-Penetrant, and Anti-inflammatory Agents.Top Curr Chem (Cham). 2024 Oct 21;382(4):33. doi: 10.1007/s41061-024-00477-6. Top Curr Chem (Cham). 2024. PMID: 39432195 Review.
-
Explainable artificial intelligence-assisted virtual screening and bioinformatics approaches for effective bioactivity prediction of phenolic cyclooxygenase-2 (COX-2) inhibitors using PubChem molecular fingerprints.Mol Divers. 2024 Aug;28(4):2099-2118. doi: 10.1007/s11030-023-10782-9. Epub 2024 Jan 10. Mol Divers. 2024. PMID: 38200203
-
Integration of pharmacophore-based virtual screening, molecular docking, ADMET analysis, and MD simulation for targeting EGFR: A comprehensive drug discovery study using commercial databases.PLoS One. 2024 Dec 9;19(12):e0311527. doi: 10.1371/journal.pone.0311527. eCollection 2024. PLoS One. 2024. PMID: 39652601 Free PMC article.
-
Mexican Native Black Bean Anthocyanin-Rich Extracts Modulate Biological Markers Associated with Inflammation.Pharmaceuticals (Basel). 2023 Jun 13;16(6):874. doi: 10.3390/ph16060874. Pharmaceuticals (Basel). 2023. PMID: 37375821 Free PMC article.
-
New Insights Into the Anticancer Effects of p-Coumaric Acid: Focus on Colorectal Cancer.Dose Response. 2023 Jan 4;21(1):15593258221150704. doi: 10.1177/15593258221150704. eCollection 2023 Jan-Mar. Dose Response. 2023. PMID: 36636631 Free PMC article. Review.
References
-
- Habauzit V, Horcajada M-N. Phenolic phytochemicals and bone. Phytochem Rev. 2008;7(2):313–344. doi:10.1007/s11101-007-9078-9 - DOI
-
- Al‐Saikhan M, Howard L, Miller JJ. Antioxidant activity and total phenolics in different genotypes of potato (Solanum tuberosum, l.). J Food Sci. 1995;60:341–343. doi:10.1111/j.1365-2621.1995.tb05668.x - DOI
-
- Izli G. Total phenolics, antioxidant capacity, colour and drying characteristics of date fruit dried with different methods. Food Sci Technol. 2017;37:139–147. doi:10.1590/1678-457x.14516 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

