COVID-19 Sepsis: Pathogenesis and Endothelial Molecular Mechanisms Based on "Two-Path Unifying Theory" of Hemostasis and Endotheliopathy-Associated Vascular Microthrombotic Disease, and Proposed Therapeutic Approach with Antimicrothrombotic Therapy
- PMID: 34103921
- PMCID: PMC8179800
- DOI: 10.2147/VHRM.S299357
COVID-19 Sepsis: Pathogenesis and Endothelial Molecular Mechanisms Based on "Two-Path Unifying Theory" of Hemostasis and Endotheliopathy-Associated Vascular Microthrombotic Disease, and Proposed Therapeutic Approach with Antimicrothrombotic Therapy
Abstract
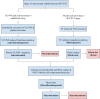
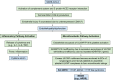
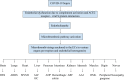
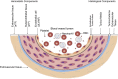
COVID-19 sepsis is characterized by acute respiratory distress syndrome (ARDS) as a consequence of pulmonary tropism of the virus and endothelial heterogeneity of the host. ARDS is a phenotype among patients with multiorgan dysfunction syndrome (MODS) due to disseminated vascular microthrombotic disease (VMTD). In response to the viral septicemia, the host activates the complement system which produces terminal complement complex C5b-9 to neutralize pathogen. C5b-9 causes pore formation on the membrane of host endothelial cells (ECs) if CD59 is underexpressed. Also, viral S protein attraction to endothelial ACE2 receptor damages ECs. Both affect ECs and provoke endotheliopathy. Disseminated endotheliopathy activates two molecular pathways: inflammatory and microthrombotic. The former releases inflammatory cytokines from ECs, which lead to inflammation. The latter initiates endothelial exocytosis of unusually large von Willebrand factor (ULVWF) multimers and FVIII from Weibel-Palade bodies. If ADAMTS13 is insufficient, ULVWF multimers activate intravascular hemostasis of ULVWF path. In activated ULVWF path, ULVWF multimers anchored to damaged endothelial cells recruit circulating platelets and trigger microthrombogenesis. This process produces "microthrombi strings" composed of platelet-ULVWF complexes, leading to endotheliopathy-associated VMTD (EA-VMTD). In COVID-19, microthrombosis initially affects the lungs per tropism causing ARDS, but EA-VMTD may orchestrate more complex clinical phenotypes, including thrombotic thrombocytopenic purpura (TTP)-like syndrome, hepatic coagulopathy, MODS and combined micro-macrothrombotic syndrome. In this pandemic, ARDS and pulmonary thromboembolism (PTE) have often coexisted. The analysis based on two hemostatic theories supports ARDS caused by activated ULVWF path is EA-VMTD and PTE caused by activated ULVWF and TF paths is macrothrombosis. The thrombotic disorder of COVID-19 sepsis is consistent with the notion that ARDS is virus-induced disseminated EA-VMTD and PTE is in-hospital vascular injury-related macrothrombosis which is not directly related to viral pathogenesis. The pathogenesis-based therapeutic approach is discussed for the treatment of EA-VMTD with antimicrothrombotic regimen and the potential need of anticoagulation therapy for coinciding macrothrombosis in comprehensive COVID-19 care.
Keywords: ADAMTS13; acute respiratory distress syndrome; ARDS; endotheliopathy; macrothrombosis; microthrombosis; multiorgan dysfunction syndrome; MODS.
© 2021 Chang.
Conflict of interest statement
The author reports no conflicts of interest in this work.
Figures

Similar articles
-
Acute Respiratory Distress Syndrome as an Organ Phenotype of Vascular Microthrombotic Disease: Based on Hemostatic Theory and Endothelial Molecular Pathogenesis.Clin Appl Thromb Hemost. 2019 Jan-Dec;25:1076029619887437. doi: 10.1177/1076029619887437. Clin Appl Thromb Hemost. 2019. PMID: 31775524 Free PMC article.
-
Pathogenesis of Two Faces of DVT: New Identity of Venous Thromboembolism as Combined Micro-Macrothrombosis via Unifying Mechanism Based on "Two-Path Unifying Theory" of Hemostasis and "Two-Activation Theory of the Endothelium".Life (Basel). 2022 Jan 31;12(2):220. doi: 10.3390/life12020220. Life (Basel). 2022. PMID: 35207507 Free PMC article. Review.
-
Vaccine-Associated Thrombocytopenia and Thrombosis: Venous Endotheliopathy Leading to Venous Combined Micro-Macrothrombosis.Medicina (Kaunas). 2021 Oct 26;57(11):1163. doi: 10.3390/medicina57111163. Medicina (Kaunas). 2021. PMID: 34833382 Free PMC article. Review.
-
Disseminated intravascular coagulation: new identity as endotheliopathy-associated vascular microthrombotic disease based on in vivo hemostasis and endothelial molecular pathogenesis.Thromb J. 2020 Oct 14;18:25. doi: 10.1186/s12959-020-00231-0. eCollection 2020. Thromb J. 2020. PMID: 33061857 Free PMC article. Review.
-
Sepsis and septic shock: endothelial molecular pathogenesis associated with vascular microthrombotic disease.Thromb J. 2019 May 30;17:10. doi: 10.1186/s12959-019-0198-4. eCollection 2019. Thromb J. 2019. PMID: 31160889 Free PMC article. Review.
Cited by
-
Novel Classification of Thrombotic Disorders Based on Molecular Hemostasis and Thrombogenesis Producing Primary and Secondary Phenotypes of Thrombosis.Biomedicines. 2022 Oct 26;10(11):2706. doi: 10.3390/biomedicines10112706. Biomedicines. 2022. PMID: 36359229 Free PMC article. Review.
-
Pathophysiology of Cardiac Injury in COVID-19 Patients with Acute Ischaemic Stroke: What Do We Know So Far?-A Review of the Current Literature.Life (Basel). 2022 Jan 6;12(1):75. doi: 10.3390/life12010075. Life (Basel). 2022. PMID: 35054468 Free PMC article. Review.
-
TTP-like syndrome and its relationship with complement activation in critically ill patients with COVID-19: A cross-sectional study.Heliyon. 2023 Jun;9(6):e17370. doi: 10.1016/j.heliyon.2023.e17370. Epub 2023 Jun 16. Heliyon. 2023. PMID: 37350773 Free PMC article.
-
Aprotinin (II): Inhalational Administration for the Treatment of COVID-19 and Other Viral Conditions.Int J Mol Sci. 2024 Jun 29;25(13):7209. doi: 10.3390/ijms25137209. Int J Mol Sci. 2024. PMID: 39000315 Free PMC article. Review.
-
Complement and COVID-19: Three years on, what we know, what we don't know, and what we ought to know.Immunobiology. 2023 May;228(3):152393. doi: 10.1016/j.imbio.2023.152393. Epub 2023 May 11. Immunobiology. 2023. PMID: 37187043 Free PMC article. Review.
References
-
- NIH. Information for Researchers: Coronaviruses. National Institute of Allergy and Infectious Disease. Available from: https://www.niaid.nih.gov/diseases-conditions/coronaviruses. Accessed April15, 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

