Removal of Cd(II), Cu(II), and Pb(II) by adsorption onto natural clay: a kinetic and thermodynamic study
- PMID: 34104050
- PMCID: PMC8164196
- DOI: 10.3906/kim-2004-82
Removal of Cd(II), Cu(II), and Pb(II) by adsorption onto natural clay: a kinetic and thermodynamic study
Abstract
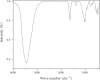
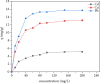
In this work, we study the elimination of three bivalent metal ions (Cd2+, Cu2+, and Pb2+) by adsorption onto natural illitic clay (AM) collected from Marrakech region in Morocco. The characterization of the adsorbent was carried out by X-ray fluorescence, Fourier transform infrared spectroscopy and X-ray diffraction. The influence of physicochemical parameters on the clay adsorption capacity for ions Cd2+, Cu2+, and Pb2+, namely the adsorbent dose, the contact time, the initial pH imposed on the aqueous solution, the initial concentration of the metal solution and the temperature, was studied. The adsorption process is evaluated by different kinetic models such as the pseudo-first-order, pseudo-second-order, and Elovich. The adsorption mechanism was determined by the use of adsorption isotherms such as Langmuir, Freundlich, and Temkin models. Experiments have shown that heavy metals adsorption kinetics onto clay follows the same order, the pseudo-second order. The isotherms of adsorption of metal cations by AM clay are satisfactorily described by the Langmuir model and the maximum adsorption capacities obtained from the natural clay, using the Langmuir isotherm model equation, are 5.25, 13.41, and 15.90 mg/g, respectively for Cd(II), Cu(II), and Pb(II) ions. Adsorption of heavy metals on clay is a spontaneous and endothermic process characterized by a disorder of the medium. The values of ΔH are greater than 40 kJ/mol, which means that the interactions between clay and heavy metals are chemical in nature.
Keywords: Clay; FTIR; adsorption; heavy metals; isotherm; thermodynamic.
Copyright © 2021 The Author(s).
Conflict of interest statement
CONFLICT OF INTEREST: none declared
Figures








References
-
- Essaadaoui Y Lebkiri A Rifi E Kadiri L Ouass A Adsorption of lead by modified Eucalyptus camaldulensis barks: equilibrium, kinetic and thermodynamic studies. Desalination and Water Treatment. 2018;111:267–277.
-
- Zhang R Zhou L Zhang F Ding Y Gao J Heavy metal pollution and assessment in the tidal flat sediments of Haizhou Bay, China. Marine Pollution Bulletin. 2013;74:403–412. - PubMed
-
- Bensalah J Habsaoui A Abbou B Kadiri L Lebkiri I Adsorption of the anionic dye methyl orange on used artificial zeolites: kinetic study and modeling of experimental data. Mediterranean Journal of Chemistry. 2019;9:311–316.
-
- Fu F Wang Q Removal of heavy metal ions from wastewaters: a review. Journal of Environmental Management. 2011;92:407–418. - PubMed
-
- Gupta VK Mittal A Gajbe V Mittal J Removal and recovery of the hazardous azo dye acid orange 7 through adsorption over waste materials: bottom ash and de-oiled soya. Industrial & Engineering Chemistry Research. 2006;45:1446–1453.
LinkOut - more resources
Full Text Sources
