Bovine Hydroxyapatite-Based Bone Scaffold with Gentamicin Accelerates Vascularization and Remodeling of Bone Defect
- PMID: 34104195
- PMCID: PMC8159631
- DOI: 10.1155/2021/5560891
Bovine Hydroxyapatite-Based Bone Scaffold with Gentamicin Accelerates Vascularization and Remodeling of Bone Defect
Abstract
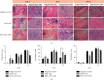
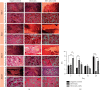
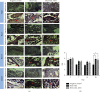
Osteomyelitis is an infectious disease which is also a major complication of bone defects. This study aims to determine the effect of bovine hydroxyapatite-gelatin-based bone implants with gentamicin as an antibiotic (BHA-GEL-GEN implant) on the regeneration of bone defects in vivo. The BHA-GEL-GEN and BHA-GEL implants were made by direct compression. In vivo study was carried out with Wistar rats. The rats were divided into three groups: negative control, BHA-GEL implant, and BHA-GEL-GEN implants. The defect model used was the burr hole defect model with diameter 2.2 mm and 2 mm deep. After 2, 7, 14, and 28 days, the rats were sacrificed. Bone integrity was carried out using X-ray radiography. Radiological examination was performed using haematoxylin and eosin (HE) staining and immunohistochemical techniques with anti-vascular endothelial growth factor (VEGF) and anti-alkaline phosphatase (ALP) antibodies. Based on the radiograph, the implanted group had accelerated bone growth in the defect area. Semiquantitative data from HE staining showed that the implanted group had accelerated migration of osteoclasts, osteoblasts, and osteocytes in the defect area. The immunoreactive score showed that the BHA-GEL-GEN group had higher VEGF expression compared to two other groups. The three groups did not provide a significant difference in ALP expression. In conclusion, the BHA-GEL-GEN implant causes accelerated bone defects repair by accelerating tissue vascularity and does not interfere with the bone remodeling process. Therefore, the BHA-GEL-GEN implant is potentially a biomedical material for osteomyelitis therapy.
Copyright © 2021 Aniek S. Budiatin et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this article.
Figures

References
-
- Budiatin A. S., Zainuddin M., Khotib J. Biocompatable composite as gentamicin delivery system for osteomyelitis and bone regeneration. International Journal of Pharmacy and Pharmaceutical Sciences. 2014;6(3):223–226.
-
- Budiatin A. S., Samirah G. M. A., Gani M. A., Nilamsari W. P., Ardianto C., Khotib J. The characterization of bovine bone-derived hydroxyapatite isolated using novel non-hazardous method. Journal of Biomimetics, Biomaterials and Biomedical Engineering. 2020;45:49–56. doi: 10.4028/www.scientific.net/jbbbe.45.49. - DOI
-
- Mohammad N. F., Othman R., Abdullah N. A., Yeoh F. Y. In vitro evaluation of mesoporous carbonated hydroxyapatite in MC3T3-E1 osteoblast cells. Procedia Chemistry. 2016;19:259–266. doi: 10.1016/j.proche.2016.03.103. - DOI
LinkOut - more resources
Full Text Sources

