Clinical outcomes, predictors of prognosis and health economics consequences in IBD patients after discontinuation of the first biological therapy
- PMID: 34104206
- PMCID: PMC8162203
- DOI: 10.1177/1756284820981216
Clinical outcomes, predictors of prognosis and health economics consequences in IBD patients after discontinuation of the first biological therapy
Abstract
Background: In real-world clinical practice, biologics in inflammatory bowel diseases (IBD) may be discontinued for a variety of reasons, including discontinuation initiated by gastroenterologists. The aims of the study are to report outcomes after discontinuation and predictors of prognosis after a minimum follow-up of 24 months; outcomes of gastroenterologist-initiated discontinuation with resulting direct cost implications on the health system were also studied.
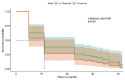
Methods: IBD patients who discontinued their first-use biologics between January 2013 and December 2016 were identified at our tertiary centre. Reasons for discontinuation and pre-defined adverse outcomes (AO) were recorded. Data were analysed using univariable and multivariable logistic regressions within a machine learning technique to predict AO. Gastroenterologist-initiated discontinuations were analysed separately, and Kaplan-Meier survival analysis performed; direct costs of AO due to discontinuation were assessed.
Results: A total of 147 patients discontinued biologics (M = 74; median age 39 years; Crohn's Disease = 110) with median follow-up of 40 months (range 24-60 months). In the total cohort, there were fewer AO among gastroenterologist-initiated discontinuations compared with patient-initiated; 54% (of the total group) had AO within 6 months. Among 59 gastroenterologist-initiated discontinuations, 23 (40%) had IBD-related AO within 6 months and 53 (90%) patients had AO by end of follow-up. Some 44 (75%) patients needed to restart biologics during follow-up, and direct costs due to AO and restart of biologics were high.
Conclusions: The proportion of patients who have AO following discontinuation of biologics is high; clinicians need to carefully consider predictors of poor prognosis and high relapse rates when discussing discontinuation. The direct costs of managing AO probably offset theoretical economic gains, especially in the era where cost of biologics is reducing. Biologics should probably be continued without interruptions in most patients who have achieved remission for the duration these remain effective and safe.
Keywords: Crohn’s disease; IBD; biologics discontinuation; biologics withdrawal; direct costs; health economics; predictors of prognosis; ulcerative colitis.
© The Author(s), 2020.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures



References
-
- Ng SC, Shi HY, Hamidi N, et al.. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2018; 390: 2769–2778. - PubMed
-
- Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol 2015; 12: 720–727. - PubMed
-
- Torres J, Bonovas S, Doherty G, et al.. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis 2020; 14: 4–22. - PubMed
-
- Rutgeerts P, Sandborn WJ, Feagan BG, et al.. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005; 353: 2462–2476. - PubMed
LinkOut - more resources
Full Text Sources

