Experience with regorafenib in the treatment of hepatocellular carcinoma
- PMID: 34104211
- PMCID: PMC8165525
- DOI: 10.1177/17562848211016959
Experience with regorafenib in the treatment of hepatocellular carcinoma
Abstract
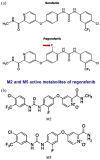
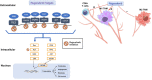
Regorafenib is a diphenylurea oral multikinase inhibitor, structurally comparable to sorafenib, which targets a variety of kinases implicated in angiogenic and tumor growth-promoting pathways. Regorafenib was the first agent to positively show significant survival advantage as a second-line therapy in patients with unresectable hepatocellular carcinoma (HCC) who had previously failed first-line treatment with sorafenib. Recent evidence has shown that its antitumor efficacy is due to a comprehensive spectrum of tumor neo-angiogenesis and proliferation inhibition and immunomodulatory effects on the tumor microenvironment, which plays a crucial role in tumor development. This review addresses the rationale and supporting evidence for regorafenib's efficacy in HCC that led to regorafenib's approval as a second-line therapy. In addition, we review proof from clinical practice studies that validate the RESORCE trial results. We discuss regorafenib's potential role in the newly emerging therapeutic strategy based on combination with immune checkpoint blockade and its possible extensibility to patient categories not enrolled in the registrative study.
Keywords: combination treatment; hepatocellular carcinoma (HCC); regorafenib; systemic treatment; tyrosine kinase inhibitor (TKI).
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: FP: (honoraria for speaker bureau, consultancy or advisory board) Alkermes, Astrazeneca, Bayer, Bracco, BMS, EISAI, GE, MSD, IPSEN, La Force Guerbet, Roche, Siemens Healthneers, Tiziana Life Sciences. Research contract with ESAOTE. FT: consultant for Bayer, Speaker bureau honoraria from MSD, Grant from Ipsen. AG, SM, AF, MR and FB have no conflict of interest to declare.
Figures

References
-
- GCO. Global Cancer Observatory, https://gco.iarc.fr/today/home (accessed 14 April 2021).
Publication types
LinkOut - more resources
Full Text Sources

