PGC-1 α Protects against Hepatic Ischemia Reperfusion Injury by Activating PPAR α and PPAR γ and Regulating ROS Production
- PMID: 34104311
- PMCID: PMC8159639
- DOI: 10.1155/2021/6677955
PGC-1 α Protects against Hepatic Ischemia Reperfusion Injury by Activating PPAR α and PPAR γ and Regulating ROS Production
Abstract
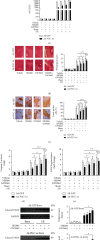
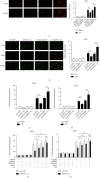
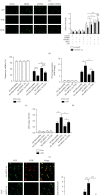
Peroxisome proliferator-activated receptors (PPARs) α and γ have been shown to be protective in hepatic ischemia/reperfusion (I/R) injury. However, the precise role of PPARγ coactivator-1α (PGC-1α), which can coactivate both of these receptors, in hepatic I/R injury, remains largely unknown. This study was designed to test our hypothesis that PGC-1α is protective during hepatic I/R injury in vitro and in vivo. Our results show that endogenous PGC-1α is basally expressed in normal livers and is moderately increased by I/R. Ectopic PGC-1α protects against hepatic I/R and hepatocyte anoxia/reoxygenation (A/R) injuries, whereas knockdown of endogenous PGC-1α aggravates such injuries, as evidenced by assessment of the levels of serum aminotransferases and inflammatory cytokines, necrosis, apoptosis, cell viability, and histological examination. The EMSA assay shows that the activation of PPARα and PPARγ is increased or decreased by the overexpression or knockdown of PGC-1α, respectively, during hepatic I/R and hepatocyte A/R injuries. In addition, the administration of specific antagonists of either PPARα (MK886) or PPARγ (GW9662) can effectively decrease the protective effect of PGC-1α against hepatic I/R and hepatocyte A/R injuries. We also demonstrate an important regulatory role of PGC-1α in reactive oxygen species (ROS) metabolism during hepatic I/R, which is correlated with the induction of ROS-detoxifying enzymes and is also dependent on the activations of PPARα and PPARγ. These data demonstrate that PGC-1α protects against hepatic I/R injury, mainly by regulating the activation of PPARα and PPARγ. Thus, PGC-1α may be a promising therapeutic target for the protection of the liver against I/R injury.
Copyright © 2021 Chaoqun Wang et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





Similar articles
-
Tumor necrosis factor and interleukin 1 decrease RXRalpha, PPARalpha, PPARgamma, LXRalpha, and the coactivators SRC-1, PGC-1alpha, and PGC-1beta in liver cells.Metabolism. 2007 Feb;56(2):267-79. doi: 10.1016/j.metabol.2006.10.007. Metabolism. 2007. PMID: 17224343 Free PMC article.
-
Silent information regulator 1 ameliorates oxidative stress injury via PGC-1α/PPARγ-Nrf2 pathway after ischemic stroke in rat.Brain Res Bull. 2022 Jan;178:37-48. doi: 10.1016/j.brainresbull.2021.11.001. Epub 2021 Nov 11. Brain Res Bull. 2022. PMID: 34774993
-
KLF5 overexpression attenuates cardiomyocyte inflammation induced by oxygen-glucose deprivation/reperfusion through the PPARγ/PGC-1α/TNF-α signaling pathway.Biomed Pharmacother. 2016 Dec;84:940-946. doi: 10.1016/j.biopha.2016.09.100. Epub 2016 Oct 17. Biomed Pharmacother. 2016. PMID: 27764756
-
Peroxisome proliferator activated receptor-alpha (PPARα) and PPAR gamma coactivator-1alpha (PGC-1α) regulation of cardiac metabolism in diabetes.Pediatr Cardiol. 2011 Mar;32(3):323-8. doi: 10.1007/s00246-011-9889-8. Epub 2011 Feb 2. Pediatr Cardiol. 2011. PMID: 21286700 Free PMC article. Review.
-
Exploiting the Therapeutic Potential of Endogenous Immunomodulatory Systems in Multiple Sclerosis-Special Focus on the Peroxisome Proliferator-Activated Receptors (PPARs) and the Kynurenines.Int J Mol Sci. 2019 Jan 19;20(2):426. doi: 10.3390/ijms20020426. Int J Mol Sci. 2019. PMID: 30669473 Free PMC article. Review.
Cited by
-
An Overview of the Role of Peroxisome Proliferator-activated Receptors in Liver Diseases.J Clin Transl Hepatol. 2023 Dec 28;11(7):1542-1552. doi: 10.14218/JCTH.2023.00334. Epub 2023 Nov 15. J Clin Transl Hepatol. 2023. PMID: 38161499 Free PMC article. Review.
-
Vitamin D improves hepatic steatosis in NAFLD via regulation of fatty acid uptake and β-oxidation.Front Endocrinol (Lausanne). 2023 Mar 22;14:1138078. doi: 10.3389/fendo.2023.1138078. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37033263 Free PMC article.
-
Reactive Oxygen Species Induce Fatty Liver and Ischemia-Reperfusion Injury by Promoting Inflammation and Cell Death.Front Immunol. 2022 Apr 29;13:870239. doi: 10.3389/fimmu.2022.870239. eCollection 2022. Front Immunol. 2022. PMID: 35572532 Free PMC article. Review.
-
San-Huang-Chai-Zhu Formula Ameliorates Liver Injury in Intrahepatic Cholestasis through Suppressing SIRT1/PGC-1α-Regulated Mitochondrial Oxidative Stress.Evid Based Complement Alternat Med. 2022 Jul 8;2022:7832540. doi: 10.1155/2022/7832540. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35845569 Free PMC article.
-
Integrative Analysis of the Roles of lncRNAs and mRNAs in Itaconate-Mediated Protection Against Liver Ischemia-Reperfusion Injury in Mice.J Inflamm Res. 2021 Sep 8;14:4519-4536. doi: 10.2147/JIR.S327467. eCollection 2021. J Inflamm Res. 2021. PMID: 34526799 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

