Up-to-Date Role of CT/MRI LI-RADS in Hepatocellular Carcinoma
- PMID: 34104640
- PMCID: PMC8180267
- DOI: 10.2147/JHC.S268288
Up-to-Date Role of CT/MRI LI-RADS in Hepatocellular Carcinoma
Abstract
Hepatocellular carcinoma (HCC) is a leading cause of mortality worldwide and a major healthcare burden in most societies. Computed tomography (CT) and magnetic resonance imaging (MRI) play a pivotal role in the medical care of patients with or at risk for hepatocellular carcinoma (HCC). When stringent imaging criteria are fulfilled, CT and MRI allow for diagnosis, staging, and assessment of response to treatment, without the need for invasive workup, and can inform clinical decision making. Owing to the central role of these imaging modalities in HCC management, standardization is essential to facilitate proper imaging technique, accurate interpretation, and clear communication among all stakeholders in both the clinical practice and research settings. The Liver Imaging Reporting and Data System (LI-RADS) is a comprehensive system that provides standardization across the continuum of HCC imaging, including ordinal probabilistic approach for reporting that directs individualized management. This review discusses the up-to-date role of CT and MRI in HCC imaging from the LI-RADS perspective. It also provides a glimpse into the future by discussing how advances in knowledge and technology are likely to enrich the LI-RADS approach.
Keywords: LI-RADS; computed tomography; hepatocellular carcinoma; magnetic resonance imaging.
© 2021 Moura Cunha et al.
Conflict of interest statement
\Dr Guilherme Moura Cunha is a member of the ACR LI-RADS steering committee; Dr. Sirlin reports grants from GE, Siemens, Philips, Bayer, Foundation of NIH, Gilead, and Pfizer (grant is to UW-Madison; UCSD is a subcontract to UW-Madison); personal consultation fees from Blade, Boehringer, and Epigenomics; consultation under the auspices of the University to AMRA, BMS, Exact Sciences, GE Digital, IBM-Watson, and Pfizer; lab service agreements from Enanta, Gilead, ICON, Intercept, Nusirt, Shire, Synageva, Takeda; royalties from Wolters Kluwer for educational material outside the submitted work; honoraria to the institution from Medscape for educational material outside the submitted work; ownership of stock options in Livivos; unpaid position in advisory board to Quantix Bio. The authors report no other conflicts of interest in this work.
Figures

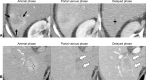


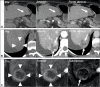
References
-
- Akinyemiju T, Abera S, Ahmed M, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol. 2017;3(12):1683–1691. doi:10.1001/jamaoncol.2017.3055 - DOI - PMC - PubMed
-
- Ludwig DR, Fraum TJ, Cannella R, et al. Expanding the Liver Imaging Reporting and Data System (LI-RADS) v2018 diagnostic population: performance and reliability of LI-RADS for distinguishing hepatocellular carcinoma (HCC) from non-HCC primary liver carcinoma in patients who do not meet strict LI-RADS high-risk criteria. HPB (Oxford). 2019;21(12):1697–1706. doi:10.1016/j.hpb.2019.04.007 - DOI - PubMed

