Incidence of SARS-CoV-2 infection according to baseline antibody status in staff and residents of 100 long-term care facilities (VIVALDI): a prospective cohort study
- PMID: 34104901
- PMCID: PMC8175048
- DOI: 10.1016/S2666-7568(21)00093-3
Incidence of SARS-CoV-2 infection according to baseline antibody status in staff and residents of 100 long-term care facilities (VIVALDI): a prospective cohort study
Abstract
Background: SARS-CoV-2 infection represents a major challenge for long-term care facilities (LTCFs) and many residents and staff are seropositive following persistent outbreaks. We aimed to investigate the association between the SARS-CoV-2 antibody status at baseline and subsequent infection in this population.
Methods: We did a prospective cohort study of SARS-CoV-2 infection in staff (aged <65 years) and residents (aged >65 years) at 100 LTCFs in England between Oct 1, 2020, and Feb 1, 2021. Blood samples were collected between June and November, 2020, at baseline, and 2 and 4 months thereafter and tested for IgG antibodies to SARS-CoV-2 nucleocapsid and spike proteins. PCR testing for SARS-CoV-2 was done weekly in staff and monthly in residents. Cox regression was used to estimate hazard ratios (HRs) of a PCR-positive test by baseline antibody status, adjusted for age and sex, and stratified by LTCF.
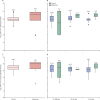
Findings: 682 residents from 86 LCTFs and 1429 staff members from 97 LTCFs met study inclusion criteria. At baseline, IgG antibodies to nucleocapsid were detected in 226 (33%) of 682 residents and 408 (29%) of 1429 staff members. 93 (20%) of 456 residents who were antibody-negative at baseline had a PCR-positive test (infection rate 0·054 per month at risk) compared with four (2%) of 226 residents who were antibody-positive at baseline (0·007 per month at risk). 111 (11%) of 1021 staff members who were antibody-negative at baseline had PCR-positive tests (0·042 per month at risk) compared with ten (2%) of 408 staff members who were antibody-positive staff at baseline (0·009 per month at risk). The risk of PCR-positive infection was higher for residents who were antibody-negative at baseline than residents who were antibody-positive at baseline (adjusted HR [aHR] 0·15, 95% CI 0·05-0·44, p=0·0006), and the risk of a PCR-positive infection was also higher for staff who were antibody-negative at baseline compared with staff who were antibody-positive at baseline (aHR 0·39, 0·19-0·82; p=0·012). 12 of 14 reinfected participants had available data on symptoms, and 11 of these participants were symptomatic. Antibody titres to spike and nucleocapsid proteins were comparable in PCR-positive and PCR-negative cases.
Interpretation: The presence of IgG antibodies to nucleocapsid protein was associated with substantially reduced risk of reinfection in staff and residents for up to 10 months after primary infection.
Funding: UK Government Department of Health and Social Care.
© 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license.
Conflict of interest statement
LS reports grants from the Department of Health and Social Care during the conduct of the study and is a member of the Social Care Working Group, which reports to the Scientific Advisory Group for Emergencies. AH is a member of the New and Emerging Respiratory Virus Threats Advisory Group at the Department of Health. All other authors declare no competing interests.
Figures


Comment in
-
COVID-19 susceptibility in long-term care facilities.Lancet Healthy Longev. 2021 Jun;2(6):e310-e311. doi: 10.1016/S2666-7568(21)00119-7. Epub 2021 Jun 3. Lancet Healthy Longev. 2021. PMID: 34104900 Free PMC article. No abstract available.
References
-
- Dora A V, Winnett A, Fulcher JA. Using serologic testing to assess the effectiveness of outbreak control efforts, serial polymerase chain reaction testing, and cohorting of positive severe acute respiratory syndrome coronavirus 2 patients in a skilled nursing facility. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1286. published online Aug 28. - DOI - PMC - PubMed
-
- Long Q-X, Liu B-Z, Deng H-J. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

