Small extracellular vesicles obtained from hypoxic mesenchymal stromal cells have unique characteristics that promote cerebral angiogenesis, brain remodeling and neurological recovery after focal cerebral ischemia in mice
- PMID: 34105014
- PMCID: PMC8187185
- DOI: 10.1007/s00395-021-00881-9
Small extracellular vesicles obtained from hypoxic mesenchymal stromal cells have unique characteristics that promote cerebral angiogenesis, brain remodeling and neurological recovery after focal cerebral ischemia in mice
Abstract
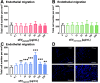
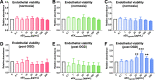
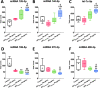
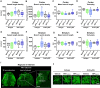
Obtained from the right cell-type, mesenchymal stromal cell (MSC)-derived small extracellular vesicles (sEVs) promote stroke recovery. Within this process, microvascular remodeling plays a central role. Herein, we evaluated the effects of MSC-sEVs on the proliferation, migration, and tube formation of human cerebral microvascular endothelial cells (hCMEC/D3) in vitro and on post-ischemic angiogenesis, brain remodeling and neurological recovery after middle cerebral artery occlusion (MCAO) in mice. In vitro, sEVs obtained from hypoxic (1% O2), but not 'normoxic' (21% O2) MSCs dose-dependently promoted endothelial proliferation, migration, and tube formation and increased post-ischemic endothelial survival. sEVs from hypoxic MSCs regulated a distinct set of miRNAs in hCMEC/D3 cells previously linked to angiogenesis, three being upregulated (miR-126-3p, miR-140-5p, let-7c-5p) and three downregulated (miR-186-5p, miR-370-3p, miR-409-3p). LC/MS-MS revealed 52 proteins differentially abundant in sEVs from hypoxic and 'normoxic' MSCs. 19 proteins were enriched (among them proteins involved in extracellular matrix-receptor interaction, focal adhesion, leukocyte transendothelial migration, protein digestion, and absorption), and 33 proteins reduced (among them proteins associated with metabolic pathways, extracellular matrix-receptor interaction, focal adhesion, and actin cytoskeleton) in hypoxic MSC-sEVs. Post-MCAO, sEVs from hypoxic MSCs increased microvascular length and branching point density in previously ischemic tissue assessed by 3D light sheet microscopy over up to 56 days, reduced delayed neuronal degeneration and brain atrophy, and enhanced neurological recovery. sEV-induced angiogenesis in vivo depended on the presence of polymorphonuclear neutrophils. In neutrophil-depleted mice, MSC-sEVs did not influence microvascular remodeling. sEVs from hypoxic MSCs have distinct angiogenic properties. Hypoxic preconditioning enhances the restorative effects of MSC-sEVs.
Keywords: Endothelial migration; Microvascular network characteristics; Microvascular remodeling; Neuronal survival; Polymorphonuclear neutrophil; Tube formation.
Conflict of interest statement
B.G. and D.M.H. hold patents for the application of extracellular vesicles for the treatment of inflammatory conditions (EP2687219A1 pending; US9877989B2 issued). B.G. is a Scientific Board member of Evox Therapeutics and Innovex Therapeutics SL. Besides, the authors report no conflicts of interest.
Figures



References
-
- Anderson JD, Johansson HJ, Graham CS, Vesterlund M, Pham MT, Bramlett CS, Montgomery EN, Mellema MS, Bardini RL, Contreras Z, Hoon M, Bauer G, Fink KD, Fury B, Hendrix KJ, Chedin F, El-Andaloussi S, Hwang B, Mulligan MS, Lehtiö J, Nolta JA. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-kappaB signaling. Stem Cells. 2016;34:601–613. doi: 10.1002/stem.2298. - DOI - PMC - PubMed
-
- Chen Z, Lai TC, Jan YH, Lin FM, Wang WC, Xiao H, Wang YT, Sun W, Cui X, Li YS, Fang T, Zhao H, Padmanabhan C, Sun R, Wang DL, Jin H, Chau GY, Huang HD, Hsiao M, Shyy JY. Hypoxia-responsive miRNAs target argonaute 1 to promote angiogenesis. J Clin Invest. 2013;123:1057–1067. doi: 10.1172/JCI65344. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EV Trust/ERA-NET EuroTransBio
- EV Trust/ERA-NET EuroTransBio
- HE3173/11-1/Deutsche Forschungsgemeinschaft
- HE3173/12-1/Deutsche Forschungsgemeinschaft
- HE3173/13-1/Deutsche Forschungsgemeinschaft
- UEFISCDI/National Authority for Science, Research and Innovation
- PN-III-P4-ID-PCE-2016-0340/National Authority for Science, Research and Innovation
- PN-III-P2-2.1-PED-2016-1013/National Authority for Science, Research and Innovation
- PN-III-P4-ID-PCE-2016-0215/National Authority for Science, Research and Innovation
- UEFISCDI/National Authority for Science, Research and Innovation
- PN-III-P4-ID-PCE-2016-0340/National Authority for Science, Research and Innovation
- PN-III-P2-2.1-PED-2016-1013/National Authority for Science, Research and Innovation
- PN-III-P4-ID-PCE-2016-0215/National Authority for Science, Research and Innovation
LinkOut - more resources
Full Text Sources
Other Literature Sources

