On the Biology of Werner's Complex
- PMID: 34105220
- PMCID: PMC8464317
- DOI: 10.1002/anie.202105019
On the Biology of Werner's Complex
Abstract
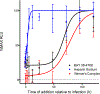
Werner's Complex, as a cationic coordination complex (CCC), has hitherto unappreciated biological properties derived from its binding affinity to highly anionic biomolecules such as glycosaminoglycans (GAGs) and nucleic acids. Competitive inhibitor and spectroscopic assays confirm the high affinity to GAGs heparin, heparan sulfate (HS), and its pentasaccharide mimetic Fondaparinux (FPX). Functional consequences of this affinity include inhibition of FPX cleavage by bacterial heparinase and mammalian heparanase enzymes with inhibition of cellular invasion and migration. Werner's Complex is a very efficient condensing agent for DNA and tRNA. In proof-of-principle for translational implications, it is demonstrated to display antiviral activity against human cytomegalovirus (HCMV) at micromolar concentrations with promising selectivity. Exploitation of non-covalent hydrogen-bonding and electrostatic interactions has motivated the unprecedented discovery of these properties, opening new avenues of research for this iconic compound.
Keywords: Antiviral efficacy; Biological Effects; DNA Binding and Condensation; Glycosaminoglycan interactions; Werner's Complex.
© 2021 Wiley-VCH GmbH.
Figures

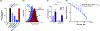

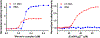



References
-
- Farrell NP. “Medicinal Inorganic Chemistry. New Perspectives and Targets for the Periodic Table.” Adv. Inorg. Chem. (AINC) 2020, Vol. 75. A thematic volume devoted to ‘Medicinal Chemistry’; Eds. van Eldik R and Sadler PJ. pp 57–86.
-
- Komeda S, Moulaei T, Woods KK, Chikuma M, Farrell N, Williams LD. J. Amer. Chem. Soc 2006, 128, 16092–16103. - PubMed
-
- Komeda S, Qu Y, Mangrum JB, Hegmans A, Williams LD, Farrell, NP NP. Inorg. Chim. Acta 2016, 452, 25–33.
-
- Calnan BJ, Tidor B, Biancalana S, Hudson D, Frankel AD. Science 1991, 252, 1167–1171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

