First report of a de novo iTTP episode associated with an mRNA-based anti-COVID-19 vaccination
- PMID: 34105244
- PMCID: PMC8236927
- DOI: 10.1111/jth.15418
First report of a de novo iTTP episode associated with an mRNA-based anti-COVID-19 vaccination
Abstract
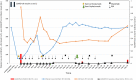
Thrombotic thrombocytopenic purpura (TTP) is a rare but potentially life-threatening thrombotic microangiopathy, characterized by disseminated thrombus formation in the microvasculature, causing severe organ failure. Immune-mediated TTP (iTTP) is occasionally described after vaccination, especially against viral agents. We report a case of a 38-year-old woman with a de novo iTTP after exposure to the mRNA-based anti-coronavirus disease 2019 (COVID-19) vaccine produced by Pfizer-BioNTech. She presented with increased bruising and petechiae starting 2 weeks after receiving the first dose of the anti-COVID-19 vaccine. Laboratory data revealed a severe ADAMTS13-deficiency in combination with a very high autoantibody titer against ADAMTS13. She was successfully treated with plasma exchange, corticosteroids, rituximab, and caplacizumab. To our knowledge, this is the first case report of iTTP after mRNA-based COVID-19 vaccination in a previously TTP-naïve patient.
Keywords: COVID-19; corticosteroids; plasmapheresis; purpura; thrombotic thrombocytopenic; vaccination.
© 2021 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- George JN. Clinical practice. Thrombotic thrombocytopenic purpura. N Engl J Med. 2006;354(18):1927‐1935. - PubMed
-
- Kremer Hovinga JA, Coppo P, Lämmle B, Moake JL, Miyata T, Vanhoorelbeke K. Thrombotic thrombocytopenic purpura. Nat Rev Dis Primers. 2017;3(1):17020. - PubMed
-
- Alwan F, Vendramin C, Liesner R, et al. Characterization and treatment of congenital thrombotic thrombocytopenic purpura. Blood. 2019;133(15):1644‐1651. - PubMed
-
- Scully M, Hunt BJ, Benjamin S, et al. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol. 2012;158(3):323‐335. - PubMed
-
- Joly BS, Coppo P, Veyradier A. An update on pathogenesis and diagnosis of thrombotic thrombocytopenic purpura. Expert Rev Hematol. 2019;12(6):383‐395. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical