Physiological and Pathological Factors Affecting Drug Delivery to the Brain by Nanoparticles
- PMID: 34105297
- PMCID: PMC8188209
- DOI: 10.1002/advs.202002085
Physiological and Pathological Factors Affecting Drug Delivery to the Brain by Nanoparticles
Abstract
The prevalence of neurological/neurodegenerative diseases, such as Alzheimer's disease is known to be increasing due to an aging population and is anticipated to further grow in the decades ahead. The treatment of brain diseases is challenging partly due to the inaccessibility of therapeutic agents to the brain. An increasingly important observation is that the physiology of the brain alters during many brain diseases, and aging adds even more to the complexity of the disease. There is a notion that the permeability of the blood-brain barrier (BBB) increases with aging or disease, however, the body has a defense mechanism that still retains the separation of the brain from harmful chemicals in the blood. This makes drug delivery to the diseased brain, even more challenging and complex task. Here, the physiological changes to the diseased brain and aged brain are covered in the context of drug delivery to the brain using nanoparticles. Also, recent and novel approaches are discussed for the delivery of therapeutic agents to the diseased brain using nanoparticle based or magnetic resonance imaging guided systems. Furthermore, the complement activation, toxicity, and immunogenicity of brain targeting nanoparticles as well as novel in vitro BBB models are discussed.
Keywords: aging brain; blood-brain barrier model; complement activation; drug delivery to the brain; immunogenicity; nanoparticles; neurodegenerative diseases.
© 2021 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
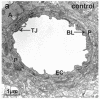



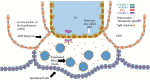
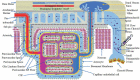
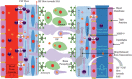



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
