Abuse-Tolerant Electrolytes for Lithium-Ion Batteries
- PMID: 34105300
- PMCID: PMC8188208
- DOI: 10.1002/advs.202003694
Abuse-Tolerant Electrolytes for Lithium-Ion Batteries
Abstract
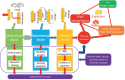
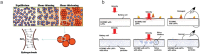
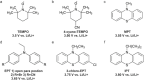
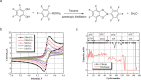
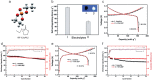
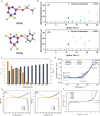
Safety issues currently limit the development of advanced lithium-ion batteries (LIBs) and this is exacerbated when they are misused or abused. The addition of small amounts of fillers or additives into common liquid electrolytes can greatly improve resistance to abuse without impairing electrochemical performance. This review discusses the recent progress in such abuse-tolerant electrolytes. It covers electrolytes with shear thickening properties for tolerating mechanical abuse, electrolytes with redox shuttle additives for suppressing electrochemical abuse, and electrolytes with flame-retardant additives for resisting thermal abuse. It aims to provide insights into the functioning of such electrolytes and the understanding of electrolyte composition-property relationship. Future perspectives, challenges, and opportunities towards practical applications are also presented.
Keywords: electrolytes; flame retardants; lithium-ion batteries; redox shuttles; shear thickening.
© 2021 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- a) Al‐Shetwi A. Q., Hannan M. A., Jern K. P., Mansur M., Mahlia T. M. I., J. Cleaner Prod. 2020, 253, 119831;
- b) Qazi A., Hussain F., Rahim N. A., Hardaker G., Alghazzawi D., Shaban K., Haruna K., IEEE Access 2019, 7, 63837;
- c) Sheibani M. R., Yousefi G. R., Latify M. A., Dolatabadi S. H., IET Renewable Power Gener. 2018, 12, 1203;
- d) Guelpa E., Bischi A., Verda V., Chertkov M., Lund H., Energy 2019, 184, 2;
- e) Khan Z., Ali S., Umar M., Kirikkaleli D., Jiao Z. L., Sci. Total Environ. 2020, 730, 10. - PubMed
-
- a) Abada S., Marlair G., Lecocq A., Petit M., Sauvant‐Moynot V., Huet F., J. Power Sources 2016, 306, 178;
- b) Di Lecce D., Verrelli R., Hassoun J., Green Chem. 2017, 19, 3442;
- c) Aaldering L. J., Leker J., Song C. H., J. Cleaner Prod. 2019, 223, 301;
- d) Yang Y. Q., Bremner S., Menictas C., Kay M., Renewable Sustainable Energy Rev. 2018, 91, 109;
- e) Qunais T., Sharma R., Karimi‐Ghartemani M., Silwal S., Khajehoddin S. A., in 2019 IEEE 28th Int. Symp. Industrial Electronics, IEEE, New York: 2019, p. 52.
-
- a) Yao Z., Tang W., Wang X., Wang C., Yang C., Fan C., J. Power Sources 2020, 448, 227456;
- b) Cao J., Xie H., Lv F., Xu N., Lee W. S. V., Ma Y., Liu Y., Cheng Z., Chen L., ACS Appl. Energy Mater. 2020, 3, 5462;
- c) Pan Y., Tzeng Y., IEEE Trans. Nanotechnol. 2019, 18, 1097;
- d) Choi J. W., Aurbach D., Nat. Rev. Mater. 2016, 1, 16013;
- e) Kumar S. B., Buckley J. P., Shi H., (Zenlabs Energy Inc.) US10056644B2, 2018.
-
- a) Rechargeable Lithium Batteries: From Fundamentals to Applications, (Ed: Franco A. A.), Woodhead Publishing, Sawston, UK: 2015;
- b) Chu S., Cui Y., Liu N., Nat. Mater. 2017, 16, 16; - PubMed
- c) Armand M., Tarascon J. M., Nature 2008, 451, 652; - PubMed
- d) Goodenough J. B., Kim Y., Chem. Mater. 2010, 22, 587;
- e) Bruce D., Haresh K., Jean‐Marie T., Science 2011, 334, 928. - PubMed
-
- a) Ramanan A., Resonance 2019, 24, 1381;
- b) Kamat P. V., ACS Energy Lett. 2019, 4, 2757; - PMC - PubMed
- c) Temming M., Lambert J., Science News for Students 2019, N.PAG;
- d) Brédas J.‐L., Buriak J. M., Caruso F., Choi K.‐S., Korgel B. A., Palacín M. R., Persson K., Reichmanis E., Schüth F., Seshadri R., Ward M. D., Chem. Mater. 2019, 31, 8577.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
