Optimizing the Start Time of Biologics in Polyarticular Juvenile Idiopathic Arthritis: A Comparative Effectiveness Study of Childhood Arthritis and Rheumatology Research Alliance Consensus Treatment Plans
- PMID: 34105312
- PMCID: PMC8518909
- DOI: 10.1002/art.41888
Optimizing the Start Time of Biologics in Polyarticular Juvenile Idiopathic Arthritis: A Comparative Effectiveness Study of Childhood Arthritis and Rheumatology Research Alliance Consensus Treatment Plans
Abstract
Objective: The optimal time to start biologics in polyarticular juvenile idiopathic arthritis (JIA) remains uncertain. The Childhood Arthritis and Rheumatology Research Alliance (CARRA) developed 3 consensus treatment plans (CTPs) for untreated polyarticular JIA to compare strategies for starting biologics.
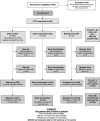
Methods: Start Time Optimization of Biologics in Polyarticular JIA (STOP-JIA) was a prospective, observational, CARRA Registry study comparing the effectiveness of 3 CTPs: 1) the step-up plan (initial nonbiologic disease-modifying antirheumatic drug [DMARD] monotherapy, adding a biologic if needed, 2) the early combination plan (DMARD and biologic started together), and 3) the biologic first plan (biologic monotherapy). The primary outcome measure was clinically inactive disease according to the provisional American College of Rheumatology (ACR) criteria, without glucocorticoids, at 12 months. Secondary outcome measures included Patient-Reported Outcomes Measurement Information System (PROMIS) pain interference and mobility scores, inactive disease as defined by the clinical Juvenile Arthritis Disease Activity Score in 10 joints (JADAS-10), and the ACR Pediatric 70 criteria (Pedi 70).
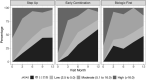
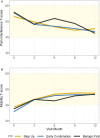
Results: Of 400 patients enrolled, 257 (64%) began the step-up plan, 100 (25%) the early combination plan, and 43 (11%) the biologic first plan. After propensity score weighting and multiple imputation, clinically inactive disease according to the ACR criteria was achieved in 37% of those on the early combination plan, 32% on the step-up plan, and 24% on the biologic first plan (P = 0.17). Inactive disease according to the clinical JADAS-10 (score ≤2.5) was also achieved in more patients on the early combination plan than the step-up plan (59% versus 43%; P = 0.03), as was ACR Pedi 70 (81% versus 62%; P = 0.008), but generalizability was limited by missing data. PROMIS measures improved in all groups, but without significant differences. Twenty serious adverse events were reported (mostly infections).
Conclusion: Achievement of clinically inactive disease without glucocorticoids did not significantly differ between groups at 12 months. While there was a significantly higher likelihood of early combination therapy achieving inactive disease according to the clinical JADAS-10 and ACR Pedi 70, these results require further exploration.
Trial registration: ClinicalTrials.gov NCT02593006.
© 2021 The Authors. Arthritis & Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures
Similar articles
-
Comparing Three Ways to Start Treatment for Patients with Polyarticular Juvenile Idiopathic Arthritis—The Stop-JIA Trial [Internet].Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2021 Mar. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2021 Mar. PMID: 38408174 Free Books & Documents. Review.
-
Bayesian comparative effectiveness study of four consensus treatment plans for initial management of systemic juvenile idiopathic arthritis: FiRst-Line Options for Systemic juvenile idiopathic arthritis Treatment (FROST).Clin Trials. 2018 Jun;15(3):268-277. doi: 10.1177/1740774518761367. Epub 2018 Mar 15. Clin Trials. 2018. PMID: 29542334 Free PMC article. Clinical Trial.
-
Improved Disease Course Associated With Early Initiation of Biologics in Polyarticular Juvenile Idiopathic Arthritis: Trajectory Analysis of a Childhood Arthritis and Rheumatology Research Alliance Consensus Treatment Plans Study.Arthritis Rheumatol. 2021 Oct;73(10):1910-1920. doi: 10.1002/art.41892. Epub 2021 Aug 27. Arthritis Rheumatol. 2021. PMID: 34105303
-
Childhood Arthritis and Rheumatology Research Alliance consensus treatment plans for new-onset polyarticular juvenile idiopathic arthritis.Arthritis Care Res (Hoboken). 2014 Jul;66(7):1063-72. doi: 10.1002/acr.22259. Arthritis Care Res (Hoboken). 2014. PMID: 24339215 Free PMC article.
-
Protocols on classification, monitoring and therapy in children's rheumatology (PRO-KIND): results of the working group Polyarticular juvenile idiopathic arthritis.Pediatr Rheumatol Online J. 2017 Nov 7;15(1):78. doi: 10.1186/s12969-017-0206-9. Pediatr Rheumatol Online J. 2017. PMID: 29116003 Free PMC article. Review.
Cited by
-
Validation of new medication use algorithms as proxies for worsening disease activity in patients with juvenile idiopathic arthritis.Pharmacoepidemiol Drug Saf. 2024 May;33(5):e5803. doi: 10.1002/pds.5803. Pharmacoepidemiol Drug Saf. 2024. PMID: 38685851 Free PMC article.
-
First-line options for systemic juvenile idiopathic arthritis treatment: an observational study of Childhood Arthritis and Rheumatology Research Alliance Consensus Treatment Plans.Pediatr Rheumatol Online J. 2022 Dec 8;20(1):113. doi: 10.1186/s12969-022-00768-6. Pediatr Rheumatol Online J. 2022. PMID: 36482434 Free PMC article.
-
Juvenile idiopathic arthritis.Nat Rev Dis Primers. 2022 Jan 27;8(1):5. doi: 10.1038/s41572-021-00332-8. Nat Rev Dis Primers. 2022. PMID: 35087087 Review.
-
Use of Patient-Reported Outcomes Measurement Information System Pediatric Measures as Clinical Trial Endpoints: Experience from a Multicenter Pragmatic Trial in Children with Crohn's Disease.J Pediatr. 2022 Mar;242:86-92.e3. doi: 10.1016/j.jpeds.2021.10.053. Epub 2021 Nov 3. J Pediatr. 2022. PMID: 34740588 Free PMC article. Clinical Trial.
-
Joint-Specific Memory and Sustained Risk for New Joint Accumulation in Autoimmune Arthritis.Arthritis Rheumatol. 2022 Nov;74(11):1851-1858. doi: 10.1002/art.42240. Epub 2022 Sep 30. Arthritis Rheumatol. 2022. PMID: 35606924 Free PMC article.
References
-
- Sacks JJ, Helmick CG, Luo YH, Ilowite NT, Bowyer S. Prevalence of and annual ambulatory health care visits for pediatric arthritis and other rheumatologic conditions in the United States in 2001–2004. Arthritis Rheum 2007;57:1439–45. - PubMed
-
- Denardo BA, Tucker LB, Miller LC, Szer IS, Schaller JG. Demography of a regional pediatric rheumatology patient population: affiliated children's arthritis centers of New England. J Rheumatol 1994;21:1553–61. - PubMed
-
- Malleson PN, Fung MY, Rosenberg AM. The incidence of pediatric rheumatic diseases: results from the Canadian Pediatric Rheumatology Association Disease Registry. J Rheumatol 1996;23:1981–7. - PubMed
-
- Oen KG, Cheang M. Epidemiology of chronic arthritis in childhood. Semin Arthritis Rheum 1996;26:575–91. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

