Fronto-parietal homotopy in resting-state functional connectivity predicts task-switching performance
- PMID: 34106305
- PMCID: PMC8843912
- DOI: 10.1007/s00429-021-02312-w
Fronto-parietal homotopy in resting-state functional connectivity predicts task-switching performance
Abstract
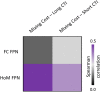
Homotopic functional connectivity reflects the degree of synchrony in spontaneous activity between homologous voxels in the two hemispheres. Previous studies have associated increased brain homotopy and decreased white matter integrity with performance decrements on different cognitive tasks across the life-span. Here, we correlated functional homotopy, both at the whole-brain level and specifically in fronto-parietal network nodes, with task-switching performance in young adults. Cue-to-target intervals (CTI: 300 vs. 1200 ms) were manipulated on a trial-by-trial basis to modulate cognitive demands and strategic control. We found that mixing costs, a measure of task-set maintenance and monitoring, were significantly correlated to homotopy in different nodes of the fronto-parietal network depending on CTI. In particular, mixing costs for short CTI trials were smaller with lower homotopy in the superior frontal gyrus, whereas mixing costs for long CTI trials were smaller with lower homotopy in the supramarginal gyrus. These results were specific to the fronto-parietal network, as similar voxel-wise analyses within a control language network did not yield significant correlations with behavior. These findings extend previous literature on the relationship between homotopy and cognitive performance to task-switching, and show a dissociable role of homotopy in different fronto-parietal nodes depending on task demands.
Keywords: Executive functions; Hemispheric asymmetries; Homotopy; Mixing costs; Resting-state fMRI; Task-switching.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures








References
-
- Allport A, Wylie G (1999) Task-switching: Positive and negative priming of task-set, In: Humphreys GW, Duncan J, Treisman A (Eds) Attention, space and action: studies in cognitive neuroscience, Oxford University Press, pp 273–296
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

