Mechanism of action of PD-1 receptor/ligand targeted cancer immunotherapy
- PMID: 34106465
- PMCID: PMC8453912
- DOI: 10.1002/eji.202048994
Mechanism of action of PD-1 receptor/ligand targeted cancer immunotherapy
Abstract
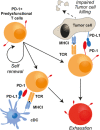
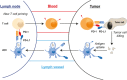
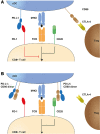
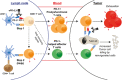
Immunotherapy targeting the Programmed Death (PD-1) receptor/ligand (L) "checkpoint" rapidly gains ground in the treatment of many cancer types. To increase treatment scope and efficacy, predictive biomarkers and rational selection of co-treatments are required. To meet these demands, we must understand PD-1 function in detail. We here outline recent insights into the regulation of the CD8+ T cell response by PD-1. The prevailing view has been that blockade of PD-1/ligand (L) interaction "reinvigorates" cytotoxic T lymphocytes (CTL) that were rendered dysfunctional in the tumor microenvironment (TME). However, this review stresses that tumors continuously communicate with adjacent draining lymph nodes (LNs) and that the PD-1 checkpoint also operates during T cell priming. We clarify the role of the PD-(L)1 system at the T cell/DC interface, where it regulates T cell receptor (TCR) signaling and CD28 costimulation and thus controls activation of tumor-specific T cells. We also highlight the importance of CD4+ T cell help during priming, which allows DCs to provide other costimulatory and cytokine signals required for optimal CTL differentiation and likely avoidance of a dysfunctional state. Therefore, we pose that PD-(L)1 blockade should exploit LN function and be combined with "help" signals to optimize CTL efficacy.
Keywords: PD-1; cytotoxic T cell; exhaustion; immunotherapy; priming.
© 2021 The Authors. European Journal of Immunology published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no commercial or financial conflict of interest.
Figures

References
-
- Schumacher, T. N. and Schreiber, R. D., Neoantigens in cancer immunotherapy. Science. 2015. 348: 69–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

