Randomized Trial of Fetal Surgery for Severe Left Diaphragmatic Hernia
- PMID: 34106556
- PMCID: PMC7613453
- DOI: 10.1056/NEJMoa2027030
Randomized Trial of Fetal Surgery for Severe Left Diaphragmatic Hernia
Abstract
Background: Observational studies have shown that fetoscopic endoluminal tracheal occlusion (FETO) has been associated with increased survival among infants with severe pulmonary hypoplasia due to isolated congenital diaphragmatic hernia on the left side, but data from randomized trials are lacking.
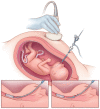
Methods: In this open-label trial conducted at centers with experience in FETO and other types of prenatal surgery, we randomly assigned, in a 1:1 ratio, women carrying singleton fetuses with severe isolated congenital diaphragmatic hernia on the left side to FETO at 27 to 29 weeks of gestation or expectant care. Both treatments were followed by standardized postnatal care. The primary outcome was infant survival to discharge from the neonatal intensive care unit. We used a group-sequential design with five prespecified interim analyses for superiority, with a maximum sample size of 116 women.
Results: The trial was stopped early for efficacy after the third interim analysis. In an intention-to-treat analysis that included 80 women, 40% of infants (16 of 40) in the FETO group survived to discharge, as compared with 15% (6 of 40) in the expectant care group (relative risk, 2.67; 95% confidence interval [CI], 1.22 to 6.11; two-sided P = 0.009). Survival to 6 months of age was identical to the survival to discharge (relative risk, 2.67; 95% CI, 1.22 to 6.11). The incidence of preterm, prelabor rupture of membranes was higher among women in the FETO group than among those in the expectant care group (47% vs. 11%; relative risk, 4.51; 95% CI, 1.83 to 11.9), as was the incidence of preterm birth (75% vs. 29%; relative risk, 2.59; 95% CI, 1.59 to 4.52). One neonatal death occurred after emergency delivery for placental laceration from fetoscopic balloon removal, and one neonatal death occurred because of failed balloon removal. In an analysis that included 11 additional participants with data that were available after the trial was stopped, survival to discharge was 36% among infants in the FETO group and 14% among those in the expectant care group (relative risk, 2.65; 95% CI, 1.21 to 6.09).
Conclusions: In fetuses with isolated severe congenital diaphragmatic hernia on the left side, FETO performed at 27 to 29 weeks of gestation resulted in a significant benefit over expectant care with respect to survival to discharge, and this benefit was sustained to 6 months of age. FETO increased the risks of preterm, prelabor rupture of membranes and preterm birth. (Funded by the European Commission and others; TOTAL ClinicalTrials.gov number, NCT01240057.).
Copyright © 2021 Massachusetts Medical Society.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
Comment in
-
Increased survival following fetoscopic endoluminal tracheal occlusion for diaphragmatic hernia.J Pediatr. 2021 Nov;238:338-342. doi: 10.1016/j.jpeds.2021.08.061. J Pediatr. 2021. PMID: 34702501 No abstract available.
-
Fetal Surgery for Severe Left Diaphragmatic Hernia.N Engl J Med. 2021 Nov 25;385(22):2111-2112. doi: 10.1056/NEJMc2115673. N Engl J Med. 2021. PMID: 34818499 No abstract available.
-
EBNEO commentary: Foetal surgery for severe left diaphragmatic hernia.Acta Paediatr. 2022 Jun;111(6):1287-1288. doi: 10.1111/apa.16299. Epub 2022 Feb 22. Acta Paediatr. 2022. PMID: 35178740 No abstract available.
References
-
- Dolk H, Loane M, Garne E. The prevalence of congenital anomalies in Europe. Adv Exp Med Biol. 2010;686:349–64. - PubMed
-
- Langham MR, Jr, Kays DW, Ledbetter DJ, Frentzen B, Sanford LL, Richards DS. Congenital diaphragmatic hernia: epidemiology and outcome. Clin Perinatol. 1996;23:671–88. - PubMed
-
- Ameis D, Khoshgoo N, Keijzer R. Abnormal lung development in congenital diaphragmatic hernia. Semin Pediatr Surg. 2017;26:123–8. - PubMed
-
- Harting MT, Lally KP. The Congenital Diaphragmatic Hernia Study Group registry update. Semin Fetal Neonatal Med. 2014;19:370–5. - PubMed
-
- American Academy of Pediatrics Section on Surgery, American Academy of Pediatrics Committee on Fetus and Newborn. Lally KP, Engle W. Postdischarge follow-up of infants with congenital diaphragmatic hernia. Pediatrics. 2008;121:627–32. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
