Diversity of trypanosomes in humans and cattle in the HAT foci Mandoul and Maro, Southern Chad-A matter of concern for zoonotic potential?
- PMID: 34106914
- PMCID: PMC8224965
- DOI: 10.1371/journal.pntd.0009323
Diversity of trypanosomes in humans and cattle in the HAT foci Mandoul and Maro, Southern Chad-A matter of concern for zoonotic potential?
Abstract
Background: African trypanosomes are parasites mainly transmitted by tsetse flies. They cause trypanosomiasis in humans (HAT) and animals (AAT). In Chad, HAT/AAT are endemic. This study investigates the diversity and distribution of trypanosomes in Mandoul, an isolated area where a tsetse control campaign is ongoing, and Maro, an area bordering the Central African Republic (CAR) where the control had not started.
Methods: 717 human and 540 cattle blood samples were collected, and 177 tsetse flies were caught. Trypanosomal DNA was detected using PCR targeting internal transcribed spacer 1 (ITS1) and glycosomal glyceraldehyde-3 phosphate dehydrogenase (gGAPDH), followed by amplicon sequencing.
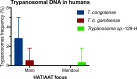
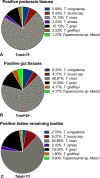
Results: Trypanosomal DNA was identified in 14 human samples, 227 cattle samples, and in tsetse. Besides T. b. gambiense, T. congolense was detected in human in Maro. In Mandoul, DNA from an unknown Trypanosoma sp.-129-H was detected in a human with a history of a cured HAT infection and persisting symptoms. In cattle and tsetse samples from Maro, T. godfreyi and T. grayi were detected besides the known animal pathogens, in addition to T. theileri (in cattle) and T. simiae (in tsetse). Furthermore, in Maro, evidence for additional unknown trypanosomes was obtained in tsetse. In contrast, in the Mandoul area, only T. theileri, T. simiae, and T. vivax DNA was identified in cattle. Genetic diversity was most prominent in T. vivax and T. theileri.
Conclusion: Tsetse control activities in Mandoul reduced the tsetse population and thus the pathogenic parasites. Nevertheless, T. theileri, T. vivax, and T. simiae are frequent in cattle suggesting transmission by other insect vectors. In contrast, in Maro, transhumance to/from Central African Republic and no tsetse control may have led to the high diversity and frequency of trypanosomes observed including HAT/AAT pathogenic species. Active HAT infections stress the need to enforce monitoring and control campaigns. Additionally, the diverse trypanosome species in humans and cattle indicate the necessity to investigate the infectivity of the unknown trypanosomes regarding their zoonotic potential. Finally, this study should be widened to other trypanosome hosts to capture the whole diversity of circulating trypanosomes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- WHO. WHO_Trypanosomiasis, human African (sleeping sickness), Feb. 2020_. https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-a.... https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-a.... Published 2020.
-
- WHO | Progress on eliminating sleeping sickness as a public health problem. WHO. Accessed December 26, 2019. http://www.who.int/trypanosomiasis_african/news/progress-on-eliminating-...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

