Lack of efficacy of hydroxychloroquine and azithromycin in patients hospitalized for COVID-19 pneumonia: A retrospective study
- PMID: 34106964
- PMCID: PMC8189518
- DOI: 10.1371/journal.pone.0252388
Lack of efficacy of hydroxychloroquine and azithromycin in patients hospitalized for COVID-19 pneumonia: A retrospective study
Abstract
Background: Hydroxychloroquine combined with azithromycin (HCQ/AZI) has initially been used against coronavirus disease-2019 (COVID-19). In this retrospective study, we assessed the clinical effects of HCQ/AZI, with a 28-days follow-up.
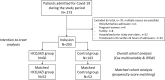
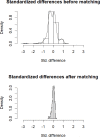
Methods: In a registry-study which included patients hospitalized for COVID-19 between March 15 and April 2, 2020, we compared patients who received HCQ/AZI to those who did not, regarding a composite outcome of mortality and mechanical ventilation with a 28-days follow-up. QT was monitored for patients treated with HCQ/AZI. Were excluded patients in intensive care units, palliative care and ventilated within 24 hours of admission. Three analyses were performed to adjust for selection bias: propensity score matching, multivariable survival, and inverse probability score weighting (IPSW) analyses.
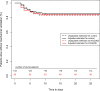
Results: Overall, 203 patients were included: 60 patients treated by HCQ/AZI and 143 control patients. During the 28-days follow-up, 32 (16.3%) patients presented the primary outcome and 23 (12.3%) patients died. Propensity-score matching identified 52 unique pairs of patients with similar characteristics. In the matched cohort (n = 104), HCQ/AZI was not associated with the primary composite outcome (log-rank p-value = 0.16). In the overall cohort (n = 203), survival and IPSW analyses also found no benefit from HCQ/AZI. In the HCQ/AZI group, 11 (18.3%) patients prolonged QT interval duration, requiring treatment cessation.
Conclusions: HCQ/AZI combination therapy was not associated with lower in-hospital mortality and mechanical ventilation rate, with a 28-days follow-up. In the HCQ/AZI group, 18.3% of patients presented a prolonged QT interval requiring treatment cessation, however, control group was not monitored for this adverse event, making comparison impossible.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al.. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clinical Infectious Diseases. 2020:ciaa237. doi: 10.1093/cid/ciaa237 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

