Treatment-related risk factors for inhibitor development in non-severe hemophilia A after 50 cumulative exposure days: A case-control study
- PMID: 34107158
- PMCID: PMC8457239
- DOI: 10.1111/jth.15419
Treatment-related risk factors for inhibitor development in non-severe hemophilia A after 50 cumulative exposure days: A case-control study
Abstract
Background: Non-severe hemophilia A patients have a life-long inhibitor risk. Yet, no studies have analyzed risk factors for inhibitor development after 50 factor VIII (FVIII) exposure days (EDs).
Objectives: This case-control study investigated treatment-related risk factors for inhibitor development in non-severe hemophilia A and assessed whether these risk factors were different for early versus late inhibitor development.
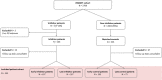
Patients/methods: Non-severe hemophilia A patients (FVIII:C 2%-40%) were selected from the INSIGHT study. Inhibitor-positive patients were defined as early (<50 EDs) or late (>50EDs) cases and matched to 1-4 inhibitor-negative controls by year of birth, cumulative number of EDs, and center/country. We investigated treatment intensity during the last 10 EDs prior to inhibitor development. Intensive treatment was defined as: surgery, peak treatment (10 consecutive EDs), and high mean FVIII dose (>45 IU/kg/ED). Odds ratios (OR) were calculated by logistic regression.
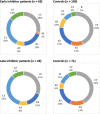
Results: Of 2709 patients, we analyzed 63 early and 26 late cases and 195 and 71 respectively matched controls. Peak treatment was associated with early and late inhibitor risk (crude OR 1.8, 95% confidence interval [CI] 1.0-3.4; 4.0, 95%CI 1.1-14.3). This association was slightly less pronounced after adjustment for mean FVIII dose. High mean FVIII dose was also associated with early and late inhibitor risk (crude OR 2.8, 95%CI 1.5-5.1; 4.5, 95%CI 1.2-16.6). Surgery increased inhibitor risk for early cases. This was less pronounced for late cases.
Conclusions: Our findings suggest that intensive FVIII treatment remains a risk factor for inhibitor development in non-severe hemophilia A after more than 50 EDs. Therefore, persistent caution is required throughout the life-time treatment course.
Keywords: anti-drug antibody; factor VIII; hemophilia A; inhibitor; risk factors.
© 2021 The Authors. Journal of Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis.
Conflict of interest statement
AA, CLE, ASV, CV, MGM, SM, and JGB have nothing to disclose. MC has received research grants from Bayer, CSL Behring, Daiichi Sankyo, Portola/Alexion, Roche, Sanquin Blood Supply, and UniQure, and consultancy and/or lecturing fees from Bayer, CSL Behring, Medcon International, MED talks, NovoNordisk, Pfizer, and Sobi. GC has received speaker, consultancy, and/or advisory fees from Ablynx, Bayer, CSL Behring, Kedrion, Novo Nordisk, Shire/Takeda, Sobi, Roche, UniQure, and Werfen, and research grants from CSL Behring, Pfizer, and Sobi. DPH has received research grants from Bayer, Octapharma, and Takeda, and advisory and/or lecturing fees from Bayer, Biomarin, Biotest, Grifols, Octapharma, Pfizer, Roche, Sanofi, Sobi, Takeda, and UniQure. CH has received consultancy and/or lecturing fees from Pfizer, Bayer, Shire/Takeda, Sobi, Biogen, CAF‐DCF, CSL Behring, LFB, Novo Nordisk, Roche, Octapharma, and Kedrion, and research funding from Pfizer, Bayer, Shire/Takeda, and Sobi. BL has received research grants from Baxter and CSL Behring. FWGL has received research support from CSL Behring, Shire/Takeda, UniQure, and Sobi and consultancy fees from UniQure, Novo Nordisk, Biomarin, and Shire/Takeda. He is a DSMB member for a study by Roche. MEM has received consultancy, advisory, and/or speaker fees from Bayer, CSL Behring, Catalyst, Biomarin, Kedrion, Grifols, Novo Nordisk, Octapharma, Pfizer, Roche, Sobi, and Takeda. JO has received honoraria and consulting fees from Chugai, CSL Behring, Grifols, Novo Nordisk, Octapharma, Pfizer, Roche, Sobi, and Shire, and grants from CSL Behring, Novo Nordisk, Octapharma, Pfizer, and Shire. CM has received personal honoraria, travel support, fees to institution for study patients, and/or unrestricted grants from Bayer, Baxalta/Shire/Takeda, Biotest, CSL Behring, Grifols, Novo Nordisk, Roche, and SOBI. KF has received unrestricted research grants from CSL Behring, Novo Nordisk, and consultancy fees from Grifols, Takeda, and Novo Nordisk. SCG has received an unrestricted research grant from Sobi.
Figures
References
-
- Blanchette VS, Key NS, Ljung LR, Manco‐Johnson MJ, van den Berg HM , Srivastava A. Definitions in hemophilia: Communication from the SSC of the ISTH. J Thromb Haemost. 2014;12(11):1935–1939. - PubMed
-
- White GC, Rosendaal F, Aledort LM, Lusher JM, Rothschild C, Ingerslev J. Definitions in hemophilia: recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the international society on thrombosis and haemostasis. Thromb Haemost. 2001;85(03):560. - PubMed
-
- Donadel‐Claeyssens S, Aronis‐Vournas S, Auerswald G, et al. Current co‐ordinated activities of the PEDNET (European Paediatric Network for Haemophilia Management). Haemophilia. 2006;12(2):124–127. - PubMed
-
- Manco‐Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535–544. - PubMed
-
- Srivastava A, Brewer AK, Mauser‐Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1‐47. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

