Efficacy of Wolbachia-Infected Mosquito Deployments for the Control of Dengue
- PMID: 34107180
- PMCID: PMC8103655
- DOI: 10.1056/NEJMoa2030243
Efficacy of Wolbachia-Infected Mosquito Deployments for the Control of Dengue
Abstract
Background: Aedes aegypti mosquitoes infected with the wMel strain of Wolbachia pipientis are less susceptible than wild-type A. aegypti to dengue virus infection.
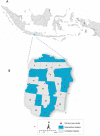
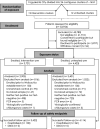
Methods: We conducted a cluster-randomized trial involving releases of wMel-infected A. aegypti mosquitoes for the control of dengue in Yogyakarta, Indonesia. We randomly assigned 12 geographic clusters to receive deployments of wMel-infected A. aegypti (intervention clusters) and 12 clusters to receive no deployments (control clusters). All clusters practiced local mosquito-control measures as usual. A test-negative design was used to assess the efficacy of the intervention. Patients with acute undifferentiated fever who presented to local primary care clinics and were 3 to 45 years of age were recruited. Laboratory testing was used to identify participants who had virologically confirmed dengue (VCD) and those who were test-negative controls. The primary end point was symptomatic VCD of any severity caused by any dengue virus serotype.
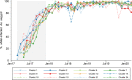
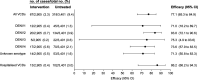
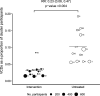
Results: After successful introgression of wMel into the intervention clusters, 8144 participants were enrolled; 3721 lived in intervention clusters, and 4423 lived in control clusters. In the intention-to-treat analysis, VCD occurred in 67 of 2905 participants (2.3%) in the intervention clusters and in 318 of 3401 (9.4%) in the control clusters (aggregate odds ratio for VCD, 0.23; 95% confidence interval [CI], 0.15 to 0.35; P = 0.004). The protective efficacy of the intervention was 77.1% (95% CI, 65.3 to 84.9) and was similar against the four dengue virus serotypes. The incidence of hospitalization for VCD was lower among participants who lived in intervention clusters (13 of 2905 participants [0.4%]) than among those who lived in control clusters (102 of 3401 [3.0%]) (protective efficacy, 86.2%; 95% CI, 66.2 to 94.3).
Conclusions: Introgression of wMel into A. aegypti populations was effective in reducing the incidence of symptomatic dengue and resulted in fewer hospitalizations for dengue among the participants. (Funded by the Tahija Foundation and others; AWED ClinicalTrials.gov number, NCT03055585; Indonesia Registry number, INA-A7OB6TW.).
Copyright © 2021 Massachusetts Medical Society.
Figures

References
-
- Simmons CP, Farrar JJ, Nguyen v V, Wills B. Dengue. N Engl J Med 2012;366:1423–32. - PubMed
-
- Ten threats to global health in 2019. 2019. (Accessed 11 September, 2020, at https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-....)
-
- Cattarino L, Rodriguez-Barraquer I, Imai N, Cummings DAT, Ferguson NM. Mapping global variation in dengue transmission intensity. Sci Transl Med 2020;12. - PubMed
-
- L’Azou M, Moureau A, Sarti E, et al. Symptomatic dengue in children in 10 Asian and Latin American countries. N Engl J Med 2016;374:1155–66. - PubMed
Publication types
MeSH terms
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
