An integrated genomic approach to dissect the genetic landscape regulating the cell-to-cell transfer of α-synuclein
- PMID: 34107263
- PMCID: PMC8207177
- DOI: 10.1016/j.celrep.2021.109189
An integrated genomic approach to dissect the genetic landscape regulating the cell-to-cell transfer of α-synuclein
Abstract
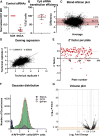
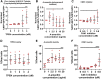
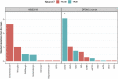
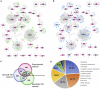
Neuropathological and experimental evidence suggests that the cell-to-cell transfer of α-synuclein has an important role in the pathogenesis of Parkinson's disease (PD). However, the mechanism underlying this phenomenon is not fully understood. We undertook a small interfering RNA (siRNA), genome-wide screen to identify genes regulating the cell-to-cell transfer of α-synuclein. A genetically encoded reporter, GFP-2A-αSynuclein-RFP, suitable for separating donor and recipient cells, was transiently transfected into HEK cells stably overexpressing α-synuclein. We find that 38 genes regulate the transfer of α-synuclein-RFP, one of which is ITGA8, a candidate gene identified through a recent PD genome-wide association study (GWAS). Weighted gene co-expression network analysis (WGCNA) and weighted protein-protein network interaction analysis (WPPNIA) show that those hits cluster in networks that include known PD genes more frequently than expected by random chance. The findings expand our understanding of the mechanism of α-synuclein spread.
Keywords: Braak hypothesis; GWAS; ITGA8; high-throughput screen; siRNA; weighted gene co-expression network analysis; weighted protein-protein network interaction analysis; α-synuclein.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests B.T.H. has a family member who works at Novartis, and owns stock in Novartis; he serves on the scientific advisory board (SAB) of Dewpoint and owns stock; he serves on an SAB or is a consultant for Avrobio, AZTherapies, Biogen, Novartis, Cell Signaling, the U.S. Department of Justice, Takeda, Vigil, W20 Group, and Seer; and his laboratory is supported by sponsored research agreements with Abbvie and F-Prime and has research grants from the National Institutes of Health, Cure Alzheimer’s Fund, Tau Consortium, and the JPB Foundation.
Figures




References
-
- Abramov A.Y., Berezhnov A.V., Fedotova E.I., Zinchenko V.P., Dolgacheva L.P. Interaction of misfolded proteins and mitochondria in neurodegenerative disorders. Biochem. Soc. Trans. 2017;45:1025–1033. - PubMed
-
- Aguzzi A., Rajendran L. The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron. 2009;64:783–790. - PubMed
-
- Aguzzi A., Heikenwalder M., Polymenidou M. Insights into prion strains and neurotoxicity. Nat. Rev. Mol. Cell Biol. 2007;8:552–561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MR/L012758/1/MRC_/Medical Research Council/United Kingdom
- MR/L501542/1/MRC_/Medical Research Council/United Kingdom
- Z01 AG000949/ImNIH/Intramural NIH HHS/United States
- G0701075/MRC_/Medical Research Council/United Kingdom
- G-1307/PUK_/Parkinson's UK/United Kingdom
- MR/J004758/1/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- MR/N008324/1/MRC_/Medical Research Council/United Kingdom
- MR/N026004/1/MRC_/Medical Research Council/United Kingdom
- MR/K01417X/1/MRC_/Medical Research Council/United Kingdom
- G-0907/PUK_/Parkinson's UK/United Kingdom
- Z01 ES101986/ImNIH/Intramural NIH HHS/United States
- G1001253/MRC_/Medical Research Council/United Kingdom
- G0901254/MRC_/Medical Research Council/United Kingdom
- 202903/Z/16/Z/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

