Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans
- PMID: 34107529
- PMCID: PMC8357629
- DOI: 10.1038/s41586-021-03681-2
Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans
Abstract
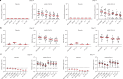
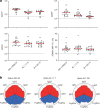
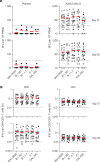
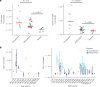
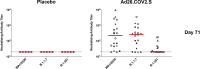
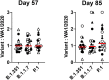
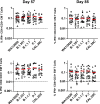
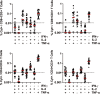
The Ad26.COV2.S vaccine1-3 has demonstrated clinical efficacy against symptomatic COVID-19, including against the B.1.351 variant that is partially resistant to neutralizing antibodies1. However, the immunogenicity of this vaccine in humans against SARS-CoV-2 variants of concern remains unclear. Here we report humoral and cellular immune responses from 20 Ad26.COV2.S vaccinated individuals from the COV1001 phase I-IIa clinical trial2 against the original SARS-CoV-2 strain WA1/2020 as well as against the B.1.1.7, CAL.20C, P.1 and B.1.351 variants of concern. Ad26.COV2.S induced median pseudovirus neutralizing antibody titres that were 5.0-fold and 3.3-fold lower against the B.1.351 and P.1 variants, respectively, as compared with WA1/2020 on day 71 after vaccination. Median binding antibody titres were 2.9-fold and 2.7-fold lower against the B.1.351 and P.1 variants, respectively, as compared with WA1/2020. Antibody-dependent cellular phagocytosis, complement deposition and natural killer cell activation responses were largely preserved against the B.1.351 variant. CD8 and CD4 T cell responses, including central and effector memory responses, were comparable among the WA1/2020, B.1.1.7, B.1.351, P.1 and CAL.20C variants. These data show that neutralizing antibody responses induced by Ad26.COV2.S were reduced against the B.1.351 and P.1 variants, but functional non-neutralizing antibody responses and T cell responses were largely preserved against SARS-CoV-2 variants. These findings have implications for vaccine protection against SARS-CoV-2 variants of concern.
© 2021. The Author(s).
Conflict of interest statement
D.H.B. is a co-inventor on related vaccine patents. M.L.G., J.S., A.M.G., D.H., F.S., M.D., J.V.H. and H.S. are employees of Janssen Pharmaceuticals and may be co-inventors on related vaccine patents. P.A.F, I.K. and H.R. are employees of Adaptive Biotechnologies. F.K. is a co-inventor on related serologic assay patents, and Mount Sinai has spun out a company, Kantaro, to market serological tests for SARS-CoV-2.
Figures





Comment in
-
Immune Responses to Recombinant Adenoviruses As Gene Therapy Vectors and COVID-19 Vaccines: A Two-Edged Sword.Hum Gene Ther. 2021 Jul;32(13-14):645-646. doi: 10.1089/hum.2021.29169.trf. Hum Gene Ther. 2021. PMID: 34283643 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

