ESGO/ISUOG/IOTA/ESGE Consensus Statement on preoperative diagnosis of ovarian tumours
- PMID: 34107646
- PMCID: PMC8291986
- DOI: 10.52054/FVVO.13.2.016
ESGO/ISUOG/IOTA/ESGE Consensus Statement on preoperative diagnosis of ovarian tumours
Abstract
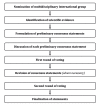
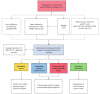
The European Society of Gynaecological Oncology (ESGO), the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG), the International Ovarian Tumour Analysis (IOTA) group and the European Society for Gynaecological Endoscopy (ESGE) jointly developed clinically relevant and evidence-based statements on the preoperative diagnosis of ovarian tumours, including imaging techniques, biomarkers and prediction models. ESGO/ISUOG/IOTA/ESGE nominated a multidisciplinary international group, including expert practising clinicians and researchers who have demonstrated leadership and expertise in the preoperative diagnosis of ovarian tumours and management of patients with ovarian cancer (19 experts across Europe). A patient representative was also included in the group. To ensure that the statements were evidence-based, the current literature was reviewed and critically appraised. Preliminary statements were drafted based on the review of the relevant literature. During a conference call, the whole group discussed each preliminary statement and a first round of voting was carried out. Statements were removed when a consensus among group members was not obtained. The voters had the opportunity to provide comments/suggestions with their votes. The statements were then revised accordingly. Another round of voting was carried out according to the same rules to allow the whole group to evaluate the revised version of the statements. The group achieved consensus on 18 statements. This Consensus Statement presents these ESGO/ISUOG/IOTA/ESGE statements on the preoperative diagnosis of ovarian tumours and the assessment of carcinomatosis, together with a summary of the evidence supporting each statement.
Conflict of interest statement
D.T.: senior investigator for FWO (Fund for Scientific Research Flanders) and research sponsored by Roche Diagnostics. KU Leuven has consultancy agreements with GE Healthcare, Samsung Healthcare, GSK and Canon. T.B.: research sponsored by Roche Diagnostics, Samsung Medison and Illumina, and grants for traveling from Samsung Medison. L.C.: advisory boards for AstraZeneca, GSK, Takeda and Roche. D.C.: advisory boards for Genmab, AstraZeneca, Roche and Sotio. N.C.: advisory boards for AstraZeneca, Seattle Genetics, Mersana and eTheRNA Immunotherapies NV, grants for traveling from Roche, Genmab and Amgen, and educational fees from MSD and Medscape Oncology. A.d.B.: advisory boards for Roche, AstraZeneca, GSK/Tesaro, BIOCAD, Clovis, Genmab/ Seattle Genetics, Pfizer and Amgen, and grants for traveling from Roche and AstraZeneca. I.V.: consulting activities for Amgen, AstraZeneca, Clovis Oncology, Carrick Therapeutics, Debiopharm International SA, Deciphera Pharmaceuticals, Elevar Therapeutics, F. Hoffmann-La Roche Ltd, Genmab, GSK, Immunogen Inc, Medical University of Vienna, Mersana, Millenium Pharmaceuticals, MSD, Novocure, Octimet Oncology NV, Oncoinvent AS, Pharmamar, Sotio a.s. Prague, Tesaro Inc, Verastem Oncology and Zentalis, contracted research (via KU Leuven) from Oncoinvent AS and Genmab, grants (corporate sponsored research) from Amgen and Roche, and accommodation/travel expenses from Amgen, MSD, Tesaro, AstraZeneca and Roche. C.F.: advisory boards for Roche, GSK, Tesaro, AZ/MSD, Clovis, Sequana and Ethicon, and grants for traveling from GSK and Roche. F.P., C.L., D.F., W.F., G.G., B.L., A.L., L.M., P.M., D.Q., A.T., V.V. and G.S. report no conflicts of interest.
Figures

References
-
- Ahmed SA, Abou-Taleb H, Yehia A, et al. The accuracy of multi-detector computed tomography and laparoscopy in the prediction of peritoneal carcinomatosis index score in primary ovarian cancer. Acad Radiol. 2019;26:1650–1658. - PubMed
-
- Alcazar JL, Pascual MA, Graupera B, et al. External validation of IOTA simple descriptors and simple rules for classifying adnexal masses. Ultrasound Obstet Gynecol. 2016;48:397–402. - PubMed
-
- Alcazar JL, Pascual MA, Olartecoechea B, et al. IOTA simple rules for discriminating between benign and malignant adnexal masses: prospective external validation. Ultrasound Obstet Gynecol. 2013;42:467–471. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
