Heterogeneity in NECTIN4 Expression Across Molecular Subtypes of Urothelial Cancer Mediates Sensitivity to Enfortumab Vedotin
- PMID: 34108177
- PMCID: PMC8634828
- DOI: 10.1158/1078-0432.CCR-20-4175
Heterogeneity in NECTIN4 Expression Across Molecular Subtypes of Urothelial Cancer Mediates Sensitivity to Enfortumab Vedotin
Abstract
Purpose: Enfortumab vedotin (EV) is an antibody-drug conjugate (ADC) targeting NECTIN4 (encoded by the PVRL4/NECTIN4 gene) approved for treatment-refractory metastatic urothelial cancer. Factors that mediate sensitivity or resistance to EV are unknown. In this study, we sought to (i) examine heterogeneity of NECTIN4 gene expression across molecular subtypes of bladder cancer and (ii) determine whether NECTIN4 expression mediates EV sensitivity or resistance.
Experimental design: Molecular subtyping and NECTIN4 expression data from seven muscle-invasive bladder cancer clinical cohorts (n = 1,915 total specimens) were used to assess NECTIN4 expression across molecular subtypes. The outcome of the transcriptomic analysis was relative NECTIN4 expression in the consensus molecular subtypes of bladder cancer. Expression of NECTIN4 was validated in bladder cancer cell lines. NECTIN4 was stably overexpressed or knocked down in basal and luminal bladder cancer cell lines and EV drug sensitivity assays were performed, as measured by cell proliferation and clonogenic assays.
Results: NECTIN4 expression is heterogenous across molecular subtypes of bladder cancer and significantly enriched in luminal subtypes. NECTIN4 expression is positively correlated with luminal markers GATA3, FOXA1, and PPARG across all cohorts. NECTIN4 expression is both necessary and sufficient for EV sensitivity in luminal and basal subtypes of urothelial bladder cancer cells. Downregulation of NECTIN4 leads to EV resistance.
Conclusions: Sensitivity to EV is mediated by expression of NECTIN4, which is enriched in luminal subtypes of bladder cancer. These findings may have implications for biomarker development, patient selection, and the inclusion of molecular subtyping in ongoing and future EV clinical trials.See related commentary by Teo and Rosenberg, p. 4950.
©2021 American Association for Cancer Research.
Figures

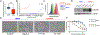

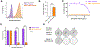
Comment in
-
NECTIN4 Heterogeneity and Molecular Diversity in Bladder Cancers: Deconstructing the Activity of An Antibody-Drug Conjugate.Clin Cancer Res. 2021 Sep 15;27(18):4950-4952. doi: 10.1158/1078-0432.CCR-21-1807. Epub 2021 Jul 19. Clin Cancer Res. 2021. PMID: 34281911
-
Urologic Oncology: Bladder, Penis, and Urethral Cancer, and Basic Principles of Oncology.J Urol. 2023 May;209(5):1025-1026. doi: 10.1097/JU.0000000000003380. Epub 2023 Feb 22. J Urol. 2023. PMID: 37026649 No abstract available.
References
-
- Hoffman-Censits JH, Choi W, Lombardo K, Hahn NM, McConkey DJ, McGuire B, et al. Expression of nectin-4 in bladder cancer with variant histology. J Clin Oncol 2020;38:546–546. 10.1200/JCO.2020.38.6_suppl.546. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

