A Phase Ib Study of Atezolizumab with Radium-223 Dichloride in Men with Metastatic Castration-Resistant Prostate Cancer
- PMID: 34108181
- PMCID: PMC8974420
- DOI: 10.1158/1078-0432.CCR-21-0063
A Phase Ib Study of Atezolizumab with Radium-223 Dichloride in Men with Metastatic Castration-Resistant Prostate Cancer
Abstract
Purpose: Men with metastatic castration-resistant prostate cancer (mCRPC) have limited treatment options after progressing on hormonal therapy and chemotherapy. Here, we evaluate the safety and efficacy of atezolizumab (anti-PD-L1) + radium-223 dichloride (radium-223) in men with mCRPC.
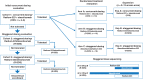
Patients and methods: This phase Ib study evaluated atezolizumab + radium-223 in men with mCRPC and bone and lymph node and/or visceral metastases that progressed after androgen pathway inhibitor treatment. Following safety assessment of concurrent dosing, 45 men were randomized 1:1:1 to concurrent or one of two staggered dosing schedules with either agent introduced one cycle before the other. This was followed by a safety-efficacy expansion cohort (randomized 1:1:1). The primary endpoints were safety and objective response rate (ORR) by RECIST 1.1. Secondary endpoints included radiographic progression-free survival (rPFS), PSA responses, and overall survival (OS).
Results: As of October 4, 2019, 44 of 45 men were evaluable. All 44 had ≥1 all-cause adverse event (AE); 23 (52.3%) had a grade 3/4 AE. Fifteen (34.1%) grade 3/4 and 3 (6.8%) grade 5 AEs were related to atezolizumab; none were related to radium-223. Confirmed ORR was 6.8% [95% confidence interval (CI), 1.4-18.7], median rPFS was 3.0 months (95% CI, 2.8-4.6), median PSA progression was 3.0 months (95% CI, 2.8-3.3), and median OS was 16.3 months (95% CI, 10.9-22.3).
Conclusions: This phase Ib study demonstrated that atezolizumab + radium-223, regardless of administration schedule, had greater toxicity than either drug alone, with no clear evidence of additional clinical benefit for patients with mCRPC and bone and lymph node and/or visceral metastases.
Trial registration: ClinicalTrials.gov NCT02814669.
©2021 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
L. Fong reports grants from AbbVie, Bavarian Nordic, Dendreon, Merck, and Roche/Genentech, as well as grants and personal fees from Bristol Myers Squibb and Janssen during the conduct of the study; L. Fong has ownership interests in Actym, Alector, Atreca, BioAlta, Bolt, Keyhole, Immunogenesis, Nutcracker, RAPT, Scribe, Senti, Soteria, and TeneoBio. M.J. Morris reports non-financial support from Roche during the conduct of the study, as well as personal fees from ORIC Pharmaceuticals and Curium outside the submitted work; M.J. Morris also reports being an uncompensated advisor to Bayer, Endocyte, Advanced Accelerator Applications, Johnson & Johnson, and Athenex. MSK received funding for clinical trials in which M.J. Morris received salary support from Bayer, Endocyte, Progenics, Corcept, Roche/Genentech, and Janssen. O. Sartor reports grants and personal fees from Advanced Accelerator Applications, AstraZeneca, Bayer, Constellation, Dendreon, Janssen, Progenics, and Sanofi; personal fees from Astellas, Blue Earth Diagnostics, Inc., Bavarian Nordic, Bristol Myers Squibb, Clarity Pharmaceuticals, Clovis, EMD Serono, Fusion, Isotopen Technologien Muenchen, Myovant, Myriad, Noria Therapeutics, Inc., Novartis, Noxopharm, POINT Biopharma, Pfizer, TeneBio, Telix, and Theragnostics; and grants from Invitae, Merck, and SOTIO during the conduct of the study. C.S. Higano reports other from F. Hoffmann-La Roche Ltd, AstraZeneca, Bayer, Clovis, Dendreon, eFFECTOR Therapeutics, Emergent, Ferring, Genentech, Medivation, Pfizer, Astellas, Aptevo, and Aragon, as well as personal fees from Astellas, Bayer, Blue Earth Diagnostics, Clovis, Dendreon, Ferring, Genentech, Carrick Therapeutics, Hinova, Janssen, Merck, Orion, Pfizer, Novartis, Tolmar, Menarini, Myovant, and Vaccitech during the conduct of the study. L. Pagliaro reports non-financial and other support from Roche/Genentech during the conduct of the study. L. Pagliaro also reports non-financial and other support from Exelixis, Pfizer, and Merck, as well as personal fees from Merck outside the submitted work. A. Alva reports grants from Genentech during the conduct of the study. A. Alva also reports grants and personal fees from Bristol Myers Squibb, Merck, and AstraZeneca, as well as grants from Progenics, Ionis, Esanik, and Arcus outside the submitted work; A. Alva is a NCCN panel member and member of ASCO and SWOG. L.J. Appleman reports grants from Roche during the conduct of the study, as well as non-financial support from Bayer outside the submitted work. W. Tan reports other from Medscape WebMD outside the submitted work. U. Vaishampayan reports grants from Genentech during the conduct of the study, as well as personal fees from Bayer, Sanofi, and Pfizer outside the submitted work. D. Tayama reports other from Genentech/Roche outside the submitted work. E.E. Kadel III reports other support from Clinuvel, Epizyme, MannKind, Merck, Roche/Genentech, and Teladoc outside the submitted work. K.C. Yuen reports personal fees from Genentech, Inc during the conduct of the study. A. Datye reports employment by F. Hoffmann-La Roche and has stock ownership in F. Hoffmann-La Roche. A.J. Armstrong reports grants from Genentech/Roche during the conduct of the study. A.J. Armstrong also reports grants and personal fees from Pfizer/Astellas, Janssen, Celgene/Bristol Myers Squibb, Merck, AstraZeneca, and Forma; grants from Constellation; and personal fees from Clovis outside the submitted work. D.P. Petrylak reports other support from Bellicum Pharmaceuticals and TYME; grants and personal fees from Advanced Accelerator Applications, AstraZeneca, Bayer, Bristol Myers Squibb, Clovis Oncology, Lilly, Pfizer, Roche, and Seattle Genetics; personal fees from Amgen, Astellas Pharma, Boehringer Ingelheim, Exelixis, Incyte, Janssen, Pharmacyclics, UroGen Pharma, Sanofi, and Celgene; and grants from Astellas Medivation, Endocyte, Genentech, Innocrin Pharma, MedImmune, Merck, Novartis, and Progenics outside the submitted work. No disclosures were reported by the other author.
Figures


References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. - PubMed
-
- Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015;16:152–60. - PubMed
-
- Pezaro CJ, Omlin AG, Altavilla A, Lorente D, Ferraldeschi R, Bianchini D, et al. Activity of cabazitaxel in castration-resistant prostate cancer progressing after docetaxel and next-generation endocrine agents. Eur Urol 2014;66:459–65. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

