Postoperative low molecular weight heparin bridging treatment for patients at high risk of arterial thromboembolism (PERIOP2): double blind randomised controlled trial
- PMID: 34108229
- PMCID: PMC8188228
- DOI: 10.1136/bmj.n1205
Postoperative low molecular weight heparin bridging treatment for patients at high risk of arterial thromboembolism (PERIOP2): double blind randomised controlled trial
Abstract
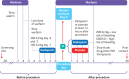
Objective: To determine the efficacy and safety of dalteparin postoperative bridging treatment versus placebo for patients with atrial fibrillation or mechanical heart valves when warfarin is temporarily interrupted for a planned procedure.
Design: Prospective, double blind, randomised controlled trial.
Setting: 10 thrombosis research sites in Canada and India between February 2007 and March 2016.
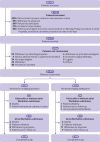
Participants: 1471 patients aged 18 years or older with atrial fibrillation or mechanical heart valves who required temporary interruption of warfarin for a procedure.
Intervention: Random assignment to dalteparin (n=821; one patient withdrew consent immediately after randomisation) or placebo (n=650) after the procedure.
Main outcome measures: Major thromboembolism (stroke, transient ischaemic attack, proximal deep vein thrombosis, pulmonary embolism, myocardial infarction, peripheral embolism, or vascular death) and major bleeding according to the International Society on Thrombosis and Haemostasis criteria within 90 days of the procedure.
Results: The rate of major thromboembolism within 90 days was 1.2% (eight events in 650 patients) for placebo and 1.0% (eight events in 820 patients) for dalteparin (P=0.64, risk difference -0.3%, 95% confidence interval -1.3 to 0.8). The rate of major bleeding was 2.0% (13 events in 650 patients) for placebo and 1.3% (11 events in 820 patients) for dalteparin (P=0.32, risk difference -0.7, 95% confidence interval -2.0 to 0.7). The results were consistent for the atrial fibrillation and mechanical heart valves groups.
Conclusions: In patients with atrial fibrillation or mechanical heart valves who had warfarin interrupted for a procedure, no significant benefit was found for postoperative dalteparin bridging to prevent major thromboembolism.
Trial registration: Clinicaltrials.gov NCT00432796.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the Canadian Institute of Health Research and Pfizer for the submitted work; SSc has received honoraria from Alnylam, Boehringer Ingelheim, Bayer HealthCare, Daiichi Sankyo, Pfizer, and Sanofi, and research support from Boehringer Ingelheim and Octapharma. None of these have any connection with the study. SMB has received consultancy fees from Leo Pharma Canada. PSW declares honoraria from Bayer Healthcare, Janssen, Sanofi, Medscape, Servier Canada, Pfizer, BMS, WebMd and grant fees from Bayer Healthcare, Pfizer/BMS in the last 3 years outside the submitted work. There are no other conflicts of interest.
Figures
References
-
- Douketis JD, Spyropoulos AC, Spencer FA, et al. . Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2 suppl):e326S-e350S. 10.1778/chest.11-2298 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
