Dysosmobacter welbionis is a newly isolated human commensal bacterium preventing diet-induced obesity and metabolic disorders in mice
- PMID: 34108237
- PMCID: PMC8862106
- DOI: 10.1136/gutjnl-2020-323778
Dysosmobacter welbionis is a newly isolated human commensal bacterium preventing diet-induced obesity and metabolic disorders in mice
Abstract
Objective: To investigate the abundance and the prevalence of Dysosmobacter welbionis J115T, a novel butyrate-producing bacterium isolated from the human gut both in the general population and in subjects with metabolic syndrome. To study the impact of this bacterium on host metabolism using diet-induced obese and diabetic mice.
Design: We analysed the presence and abundance of the bacterium in 11 984 subjects using four human cohorts (ie, Human Microbiome Project, American Gut Project, Flemish Gut Flora Project and Microbes4U). Then, we tested the effects of daily oral gavages with live D. welbionis J115T on metabolism and several hallmarks of obesity, diabetes, inflammation and lipid metabolism in obese/diabetic mice.
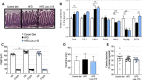
Results: This newly identified bacterium was detected in 62.7%-69.8% of the healthy population. Strikingly, in obese humans with a metabolic syndrome, the abundance of Dysosmobacter genus correlates negatively with body mass index, fasting glucose and glycated haemoglobin. In mice, supplementation with live D. welbionis J115T, but not with the pasteurised bacteria, partially counteracted diet-induced obesity development, fat mass gain, insulin resistance and white adipose tissue hypertrophy and inflammation. In addition, live D. welbionis J115T administration protected the mice from brown adipose tissue inflammation in association with increased mitochondria number and non-shivering thermogenesis. These effects occurred with minor impact on the mouse intestinal microbiota composition.
Conclusions: These results suggest that D. welbionis J115T directly and beneficially influences host metabolism and is a strong candidate for the development of next-generation beneficial bacteria targeting obesity and associated metabolic diseases.
Keywords: intestinal microbiology; obesity; probiotics.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: PDC is cofounder of A-Mansia biotech. TLR and PDC are inventors on patent applications dealing with the use bacteria in the treatment of obesity and related disorders.
Figures




References
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
