Therapeutic liver repopulation by transient acetaminophen selection of gene-modified hepatocytes
- PMID: 34108249
- PMCID: PMC9094690
- DOI: 10.1126/scitranslmed.abg3047
Therapeutic liver repopulation by transient acetaminophen selection of gene-modified hepatocytes
Abstract
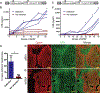
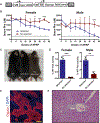
Gene therapy by integrating vectors is promising for monogenic liver diseases, especially in children where episomal vectors remain transient. However, reaching the therapeutic threshold with genome-integrating vectors is challenging. Therefore, we developed a method to expand hepatocytes bearing therapeutic transgenes. The common fever medicine acetaminophen becomes hepatotoxic via cytochrome p450 metabolism. Lentiviral vectors with transgenes linked in cis to a Cypor shRNA were administered to neonatal mice. Hepatocytes lacking the essential cofactor of Cyp enzymes, NADPH-cytochrome p450 reductase (Cypor), were selected in vivo by acetaminophen administration, replacing up to 50% of the hepatic mass. Acetaminophen treatment of the mice resulted in over 30-fold expansion of transgene-bearing hepatocytes and achieved therapeutic thresholds in hemophilia B and phenylketonuria. We conclude that therapeutically modified hepatocytes can be selected safely and efficiently in preclinical models with a transient regimen of moderately hepatotoxic acetaminophen.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Competing interests:
OHSU and M.G. have a significant financial interest in Yecuris Corporation, a company that may have a commercial interest in the results of this research and technology. M.G. is a paid consultant of Yecuris Corp., LogicBio Therapeutics, and Ambys Medicines. A patent application on the APAP selection technology described herein has been filed by OHSU (Title: Methods of Gene Therapy. Filing number: PCT/US19/29890. M.G. and A.T. are coinventors). These potential conflicts of interest have been reviewed and managed by OHSU.
Figures
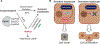


Comment in
-
From pain to gain: Leveraging acetaminophen in hepatocyte transplantation for phenylketonuria.Hepatology. 2024 May 1;79(5):973-975. doi: 10.1097/HEP.0000000000000713. Epub 2023 Dec 12. Hepatology. 2024. PMID: 38085850 No abstract available.
References
-
- Palaschak B, Herzog RW, Markusic DM, AAV-mediated gene delivery to the liver: Overview of current technologies and methods. Methods Mol. Biol 1950, 333–360 (2019). - PubMed
-
- Chirmule N, Propert KJ, Magosin SA, Qian Y, Qian R, Wilson JM, Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther 6, 1574–1583 (1999). - PubMed
-
- Cunningham SC, Dane AP, Spinoulas A, Alexander IE, Gene delivery to the juvenile mouse liver using AAV2/8 vectors. Mol. Ther 16, 1081–1088 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

