Comparison of SARS-CoV-2 Antibody Responses and Seroconversion in COVID-19 Patients Using Twelve Commercial Immunoassays
- PMID: 34108285
- PMCID: PMC8203431
- DOI: 10.3343/alm.2021.41.6.577
Comparison of SARS-CoV-2 Antibody Responses and Seroconversion in COVID-19 Patients Using Twelve Commercial Immunoassays
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody assays have high clinical utility in managing the pandemic. We compared antibody responses and seroconversion of coronavirus disease 2019 (COVID-19) patients using different immunoassays.
Methods: We evaluated 12 commercial immunoassays, including three automated chemiluminescent immunoassays (Abbott, Roche, and Siemens), three enzyme immunoassays (Bio-Rad, Euroimmun, and Vircell), five lateral flow immunoassays (Boditech Med, SD biosensor, PCL, Sugentech, and Rapigen), and one surrogate neutralizing antibody assay (GenScript) in sequential samples from 49 COVID-19 patients and 10 seroconversion panels.
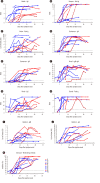
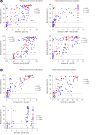
Results: The positive percent agreement (PPA) of assays for a COVID-19 diagnosis ranged from 84.0% to 98.5% for all samples (>14 days after symptom onset), with IgM or IgA assays showing higher PPAs. Seroconversion responses varied across the assay type and disease severity. Assays targeting the spike or receptor-binding domain protein showed a tendency for early seroconversion detection and higher index values in patients with severe disease. Index values from SARS-CoV-2 binding antibody assays (three automated assays, one LFIA, and three EIAs) showed moderate to strong correlations with the neutralizing antibody percentage (r=0.517-0.874), and stronger correlations in patients with severe disease and in assays targeting spike protein. Agreement among the 12 assays was good (74.3%-96.4%) for detecting IgG or total antibodies.
Conclusions: Positivity rates and seroconversion of SARS-CoV-2 antibodies vary depending on the assay kits, disease severity, and antigen target. This study contributes to a better understanding of antibody response in symptomatic COVID-19 patients using currently available assays.
Keywords: Correlation; Disease severity; Immunoassays; Neutralizing antibody; Positive percent agreement; SARS-CoV-2 antibody; Seroconversion.
Conflict of interest statement
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of the paper. The companies had no role in the design of this study, data collection, data analyses, data interpretation, writing of the manuscript, or the decision to publish the results.
Figures
Similar articles
-
Antibody response against SARS-CoV-2 spike protein and nucleoprotein evaluated by four automated immunoassays and three ELISAs.Clin Microbiol Infect. 2020 Nov;26(11):1557.e1-1557.e7. doi: 10.1016/j.cmi.2020.07.038. Epub 2020 Jul 31. Clin Microbiol Infect. 2020. PMID: 32745595 Free PMC article.
-
Clinical diagnostic performance evaluation of five immunoassays for antibodies to SARS-CoV-2 diagnosis in a real-life routine care setting.Pan Afr Med J. 2021 May 3;39:3. doi: 10.11604/pamj.2021.39.3.26471. eCollection 2021. Pan Afr Med J. 2021. PMID: 34178231 Free PMC article.
-
Diagnostic performance of four SARS-CoV-2 antibody assays in patients with COVID-19 or with bacterial and non-SARS-CoV-2 viral respiratory infections.Eur J Clin Microbiol Infect Dis. 2021 Sep;40(9):1983-1997. doi: 10.1007/s10096-021-04285-4. Epub 2021 Jun 9. Eur J Clin Microbiol Infect Dis. 2021. PMID: 34109500 Free PMC article.
-
Increasing the Efficiency of a National Laboratory Response to COVID-19: a Nationwide Multicenter Evaluation of 47 Commercial SARS-CoV-2 Immunoassays by 41 Laboratories.J Clin Microbiol. 2021 Aug 18;59(9):e0076721. doi: 10.1128/JCM.00767-21. Epub 2021 Aug 18. J Clin Microbiol. 2021. PMID: 34191578 Free PMC article. Review.
-
Challenges in the Detection of SARS-CoV-2: Evolution of the Lateral Flow Immunoassay as a Valuable Tool for Viral Diagnosis.Biosensors (Basel). 2022 Sep 5;12(9):728. doi: 10.3390/bios12090728. Biosensors (Basel). 2022. PMID: 36140114 Free PMC article. Review.
Cited by
-
Performance Evaluation of Three Antibody Binding Assays, a Neutralizing Antibody Assay, and an Interferon-Gamma Release Assay for SARS-CoV-2 According to Vaccine Type in Vaccinated Group.Diagnostics (Basel). 2023 Dec 18;13(24):3688. doi: 10.3390/diagnostics13243688. Diagnostics (Basel). 2023. PMID: 38132272 Free PMC article.
-
Longitudinal Analysis of SARS-CoV-2-Specific Cellular and Humoral Immune Responses and Breakthrough Infection following BNT162b2/BNT162b2/BNT162b2 and ChAdOx1/ChAdOx1/BNT162b2 Vaccination: A Prospective Cohort in Naive Healthcare Workers.Vaccines (Basel). 2023 Oct 19;11(10):1613. doi: 10.3390/vaccines11101613. Vaccines (Basel). 2023. PMID: 37897015 Free PMC article.
-
Humoral immune response to COVID-19 vaccination in diabetes is age-dependent but independent of type of diabetes and glycaemic control: The prospective COVAC-DM cohort study.Diabetes Obes Metab. 2022 May;24(5):849-858. doi: 10.1111/dom.14643. Epub 2022 Jan 24. Diabetes Obes Metab. 2022. PMID: 34984802 Free PMC article.
-
Humoral Response Kinetics and Cross-Immunity in Hospitalized Patients with SARS-CoV-2 WT, Delta, or Omicron Infections: A Comparison between Vaccinated and Unvaccinated Cohorts.Vaccines (Basel). 2023 Dec 1;11(12):1803. doi: 10.3390/vaccines11121803. Vaccines (Basel). 2023. PMID: 38140207 Free PMC article.
-
Recent Developments in SARS-CoV-2 Neutralizing Antibody Detection Methods.Curr Med Sci. 2021 Dec;41(6):1052-1064. doi: 10.1007/s11596-021-2470-7. Epub 2021 Dec 21. Curr Med Sci. 2021. PMID: 34935114 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

