Menopause impacts human brain structure, connectivity, energy metabolism, and amyloid-beta deposition
- PMID: 34108509
- PMCID: PMC8190071
- DOI: 10.1038/s41598-021-90084-y
Menopause impacts human brain structure, connectivity, energy metabolism, and amyloid-beta deposition
Abstract
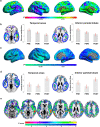
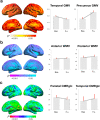
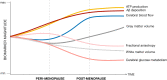
All women undergo the menopause transition (MT), a neuro-endocrinological process that impacts aging trajectories of multiple organ systems including brain. The MT occurs over time and is characterized by clinically defined stages with specific neurological symptoms. Yet, little is known of how this process impacts the human brain. This multi-modality neuroimaging study indicates substantial differences in brain structure, connectivity, and energy metabolism across MT stages (pre-menopause, peri-menopause, and post-menopause). These effects involved brain regions subserving higher-order cognitive processes and were specific to menopausal endocrine aging rather than chronological aging, as determined by comparison to age-matched males. Brain biomarkers largely stabilized post-menopause, and gray matter volume (GMV) recovered in key brain regions for cognitive aging. Notably, GMV recovery and in vivo brain mitochondria ATP production correlated with preservation of cognitive performance post-menopause, suggesting adaptive compensatory processes. In parallel to the adaptive process, amyloid-β deposition was more pronounced in peri-menopausal and post-menopausal women carrying apolipoprotein E-4 (APOE-4) genotype, the major genetic risk factor for late-onset Alzheimer's disease, relative to genotype-matched males. These data show that human menopause is a dynamic neurological transition that significantly impacts brain structure, connectivity, and metabolic profile during midlife endocrine aging of the female brain.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

