Organosilicon uptake by biological membranes
- PMID: 34108634
- PMCID: PMC8190035
- DOI: 10.1038/s42003-021-02155-5
Organosilicon uptake by biological membranes
Erratum in
-
Author Correction: Organosilicon uptake by biological membranes.Commun Biol. 2021 Jun 23;4(1):812. doi: 10.1038/s42003-021-02338-0. Commun Biol. 2021. PMID: 34162995 Free PMC article. No abstract available.
-
Publisher Correction: Organosilicon uptake by biological membranes.Commun Biol. 2021 Jun 23;4(1):813. doi: 10.1038/s42003-021-02344-2. Commun Biol. 2021. PMID: 34163005 Free PMC article. No abstract available.
Abstract
Organosilicon compounds are ubiquitous in everyday use. Application of some of these compounds in food, cosmetics and pharmaceuticals is widespread on the assumption that these materials are not systemically absorbed. Here the interactions of various organosilicon compounds (simeticone, hexamethyldisilazane and polydimethylsiloxane) with cell membranes and models thereof were characterized with a range of analytical techniques, demonstrating that these compounds were retained in or on the cell membrane. The increasing application of organosilicon compounds as replacement of other plastics calls for a better awareness and understanding of these interactions. Moreover, with many developments in biotechnology relying on organosilicon materials, it becomes important to scrutinize the potential effect that silicone leaching may have on biological systems.
Conflict of interest statement
The authors declare the following competing interests: C.O. is managing director of Hybriscan Technologies B.V. which partially funded the research. Hybriscan Technologies B.V. has no financial or non-financial competing interest in this study. All remaining authors declare no competing interests.
Figures
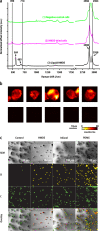
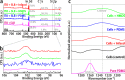

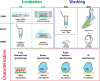
References
-
- Linti G. Organosilicon chemistry V. From molecules to materials. By Norbert Auner and Johann Weis. Angew. Chem. Int. Ed. 2004;43:2744. doi: 10.1002/anie.200385121. - DOI
-
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO Technical Report Series 966 Evaluation of certain food additives and contaminants. World Health Organ10.1016/S0140-6736(02)11326-2 (2011).
-
- Metcalf TJ, Irons TG, Sher LD, Young PC. Simethicone in the treatment of infant colic: a randomized, placebo-controlled, multicenter trial. Pediatrics. 1994;94:29–34. - PubMed
-
- Part 332-antiflatulent products for over-the-counter human use. Fed. Regist. 39, 19877 (1974).
Publication types
LinkOut - more resources
Full Text Sources

