First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland
- PMID: 34108714
- PMCID: PMC8282499
- DOI: 10.1038/s41591-021-01408-4
First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland
Abstract
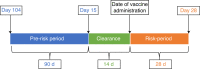
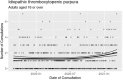
Reports of ChAdOx1 vaccine-associated thrombocytopenia and vascular adverse events have led to some countries restricting its use. Using a national prospective cohort, we estimated associations between exposure to first-dose ChAdOx1 or BNT162b2 vaccination and hematological and vascular adverse events using a nested incident-matched case-control study and a confirmatory self-controlled case series (SCCS) analysis. An association was found between ChAdOx1 vaccination and idiopathic thrombocytopenic purpura (ITP) (0-27 d after vaccination; adjusted rate ratio (aRR) = 5.77, 95% confidence interval (CI), 2.41-13.83), with an estimated incidence of 1.13 (0.62-1.63) cases per 100,000 doses. An SCCS analysis confirmed that this was unlikely due to bias (RR = 1.98 (1.29-3.02)). There was also an increased risk for arterial thromboembolic events (aRR = 1.22, 1.12-1.34) 0-27 d after vaccination, with an SCCS RR of 0.97 (0.93-1.02). For hemorrhagic events 0-27 d after vaccination, the aRR was 1.48 (1.12-1.96), with an SCCS RR of 0.95 (0.82-1.11). A first dose of ChAdOx1 was found to be associated with small increased risks of ITP, with suggestive evidence of an increased risk of arterial thromboembolic and hemorrhagic events. The attenuation of effect found in the SCCS analysis means that there is the potential for overestimation of the reported results, which might indicate the presence of some residual confounding or confounding by indication. Public health authorities should inform their jurisdictions of these relatively small increased risks associated with ChAdOx1. No positive associations were seen between BNT162b2 and thrombocytopenic, thromboembolic and hemorrhagic events.
© 2021. The Author(s).
Conflict of interest statement
A.S. is a member of the Scottish Government Chief Medical Officer’s COVID-19 Advisory Group and the New and Emerging Respiratory Virus Threats Risk Stratification Subgroup and AstraZeneca’s COVID-19 Thrombocytopenia Task Force; all roles are remunerated to A.S. or his institution. C.R.S. declares funding from the Medical Research Council, the National Institute for Health Research, the Chief Scientist Office and the New Zealand Ministry for Business, Innovation and Employment and Health Research Council during the conduct of this study. S.V.K. is co-chair of the Scottish Government’s Expert Reference Group on COVID-19 and Ethnicity, is a member of the Scientific Advisory Group on Emergencies subgroup on ethnicity and acknowledges funding from an NHS Research Scotland Senior Clinical Fellowship, the Medical Research Council and the Chief Scientist Office. C.R. is a member of the Scottish Government Chief Medical Officer’s COVID-19 Advisory Group, the Scientific Pandemic Influenza Group on Modelling and the Medicines & Healthcare products Regulatory Agency’s Vaccine Benefit and Risk Working Group. H.R.S. is an advisor to the Scottish Parliament’s COVID-19 Committee. All other authors report no financial conflicts of interest.
Figures



Comment in
-
COVID-19 vaccination and immune thrombocytopenia.Nat Med. 2021 Jul;27(7):1145-1146. doi: 10.1038/s41591-021-01419-1. Nat Med. 2021. PMID: 34108715 No abstract available.
References
-
- Joint Committee on Vaccination and Immunisation. Priority groups for coronavirus (COVID-19) vaccination: advice from the JCVI, 30 December 2020. https://www.gov.uk/ government/publications/ priority-groups-for- corona... (2020).

