Impact of vaccination on new SARS-CoV-2 infections in the United Kingdom
- PMID: 34108716
- PMCID: PMC8363500
- DOI: 10.1038/s41591-021-01410-w
Impact of vaccination on new SARS-CoV-2 infections in the United Kingdom
Abstract
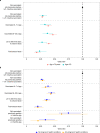
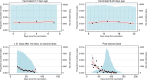
The effectiveness of COVID-19 vaccination in preventing new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections in the general community is still unclear. Here, we used the Office for National Statistics COVID-19 Infection Survey-a large community-based survey of individuals living in randomly selected private households across the United Kingdom-to assess the effectiveness of the BNT162b2 (Pfizer-BioNTech) and ChAdOx1 nCoV-19 (Oxford-AstraZeneca; ChAdOx1) vaccines against any new SARS-CoV-2 PCR-positive tests, split according to self-reported symptoms, cycle threshold value (<30 versus ≥30; as a surrogate for viral load) and gene positivity pattern (compatible with B.1.1.7 or not). Using 1,945,071 real-time PCR results from nose and throat swabs taken from 383,812 participants between 1 December 2020 and 8 May 2021, we found that vaccination with the ChAdOx1 or BNT162b2 vaccines already reduced SARS-CoV-2 infections ≥21 d after the first dose (61% (95% confidence interval (CI) = 54-68%) versus 66% (95% CI = 60-71%), respectively), with greater reductions observed after a second dose (79% (95% CI = 65-88%) versus 80% (95% CI = 73-85%), respectively). The largest reductions were observed for symptomatic infections and/or infections with a higher viral burden. Overall, COVID-19 vaccination reduced the number of new SARS-CoV-2 infections, with the largest benefit received after two vaccinations and against symptomatic and high viral burden infections, and with no evidence of a difference between the BNT162b2 and ChAdOx1 vaccines.
© 2021. The Author(s).
Conflict of interest statement
All authors have completed the International Committee of Medical Journal Editors uniform disclosure from at
Figures




References
-
- Vaccine BNT162b2—Conditions of Authorisation Under Regulation 174 (Medicines and Healthcare Products Regulatory Agency, 2020); https://www.gov.uk/government/publications/regulatory-approval-of-pfizer...
-
- Regulatory Approval of COVID-19 Vaccine AstraZeneca (Medicines and Healthcare Products Regulatory Agency, 2020); https://www.gov.uk/government/publications/regulatory-approval-of-covid-...
-
- Regulatory Approval of COVID-19 Vaccine Moderna (Medicines and Healthcare Products Regulatory Agency, 2021); https://www.gov.uk/government/publications/regulatory-approval-of-covid-...
-
- Joint Committee on Vaccination and Immunisation: Advice on Priority Groups for COVID-19 Vaccination, 30 December 2020 (Joint Committee on Vaccination and Immunisation, 2020); https://www.gov.uk/government/publications/priority-groups-for-coronavir...
-
- Vaccinations in United Kingdom (Public Health England, 2021); https://coronavirus.data.gov.uk/details/vaccinations
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

