Early Exanthema Upon Vemurafenib Plus Cobimetinib Is Associated With a Favorable Treatment Outcome in Metastatic Melanoma: A Retrospective Multicenter DeCOG Study
- PMID: 34109122
- PMCID: PMC8183381
- DOI: 10.3389/fonc.2021.672172
Early Exanthema Upon Vemurafenib Plus Cobimetinib Is Associated With a Favorable Treatment Outcome in Metastatic Melanoma: A Retrospective Multicenter DeCOG Study
Abstract
Background: The combination of BRAF and MEK inhibitors has become standard of care in the treatment of metastatic BRAF V600-mutated melanoma. Clinical factors for an early prediction of tumor response are rare. The present study investigated the association between the development of an early exanthema induced by vemurafenib or vemurafenib plus cobimetinib and therapy outcome.
Methods: This multicenter retrospective study included patients with BRAF V600-mutated irresectable AJCC-v8 stage IIIC/D to IV metastatic melanoma who received treatment with vemurafenib (VEM) or vemurafenib plus cobimetinib (COBIVEM). The development of an early exanthema within six weeks after therapy start and its grading according to CTCAEv4.0 criteria was correlated to therapy outcome in terms of best overall response, progression-free (PFS), and overall survival (OS).
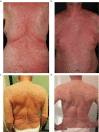
Results: A total of 422 patients from 16 centers were included (VEM, n=299; COBIVEM, n=123). 20.4% of VEM and 43.1% of COBIVEM patients developed an early exanthema. In the VEM cohort, objective responders (CR/PR) more frequently presented with an early exanthema than non-responders (SD/PD); 59.0% versus 38.7%; p=0.0027. However, median PFS and OS did not differ between VEM patients with or without an early exanthema (PFS, 6.9 versus 6.0 months, p=0.65; OS, 11.0 versus 12.4 months, p=0.69). In the COBIVEM cohort, 66.0% of objective responders had an early exanthema compared to 54.3% of non-responders (p=0.031). Median survival times were significantly longer for patients who developed an early exanthema compared to patients who did not (PFS, 9.7 versus 5.6 months, p=0.013; OS, not reached versus 11.6 months, p=0.0061). COBIVEM patients with a mild early exanthema (CTCAEv4.0 grade 1-2) had a superior survival outcome as compared to COBIVEM patients with a severe (CTCAEv4.0 grade 3-4) or non early exanthema, respectively (p=0.047). This might be caused by the fact that 23.6% of patients with severe exanthema underwent a dose reduction or discontinuation of COBIVEM compared to only 8.9% of patients with mild exanthema.
Conclusions: The development of an early exanthema within 6 weeks after treatment start indicates a favorable therapy outcome upon vemurafenib plus cobimetinib. Patients presenting with an early exanthema should therefore be treated with adequate supportive measures to provide that patients can stay on treatment.
Keywords: BRAF/MEK inhibition; cobimetinib; melanoma; skin toxicity; therapy outcome; vemurafenib.
Copyright © 2021 Kähler, Gutzmer, Meier, Zimmer, Heppt, Gesierich, Thoms, Utikal, Hassel, Loquai, Pföhler, Heinzerling, Kaatz, Göppner, Pflugfelder, Bohne, Satzger, Reinhardt, Placke, Schadendorf and Ugurel.
Conflict of interest statement
KK has served as consultant or/and has received honoraria from Amgen, Roche, Bristol Myers Squibb, Merck Sharp and Dohme, Pierre Fabre, and Novartis, and received travel support from Amgen, Merck Sharp and Dohme, Bristol Myers Squibb, Amgen, Pierre Fabre, Medac, and Novartis. RG received honoraria for lectures and advisory boards, research support and meeting support from Almirall Hermal, Amgen, Astra Zeneca, Bristol Myers Squibb, Leo, Merck Serono, Merck Sharp and Dohme, Pierre Fabre, Roche, Sanofi Genzyme, Regeneron, Sun Pharma, Takeda, Pfizer, Novartis, Johnson&Johnson, 4SC, and Incyte. FM has received travel support or/and speaker’s fees or/and advisor’s honoraria by Novartis, Roche, BMS, MSD and Pierre Fabre, and research funding from Novartis and Roche. LZ has served as consultant and/or has received honoraria from Roche, Bristol Myers Squibb, Merck Sharp and Dohme, Novartis, Pierre Fabre, and Sanofi, and received travel support from Bristol Myers Squibb, Merck Sharp and Dohme, Amgen, Pierre Fabre, and Novartis. MH has received consultant and/or speaker honoraria form Bristol Myers Squibb, Novartis, Merck Sharp and Dohme, Sanofi, Roche and travel support from Novartis, and Bristol Myers Squibb. AG reports speakers honoraria from Bristol Myers Squibb, Merck Sharp and Dohme, and Roche, advisory board honoraria from Bristol Myers Squibb, Novartis, Merck Sharp and Dohme, Pierre Fabre, Pfizer, Roche and Sanofi Genzyme, and travel support from Bristol Myers Squibb, Merck Sharp and Dohme, Novartis, and Roche. K-MT received honoraria for lectures and advisory boards from Bristol-Myers Squibb, Roche, Novartis, Merck Sharp and Dohme, Pierre Fabre, LEO, Galderma, AbbVie, La Roche-Posay and Candela, and travel support from Bristol-Myers Squibb, Roche, Novartis, Merck Sharp and Dohme, Pierre Fabre, LEO, and Candela. JU is on the advisory board or has received honoraria and travel support from Amgen, Bristol Myers Squibb, GSK, LeoPharma, Merck Sharp and Dohme, Novartis, Pierre Fabre, Roche, Sanofi outside the submitted work. JH reports speakers honoraria from Bristol Myers Squibb, Novartis, Merck Sharp and Dohme, and Roche, advisory board honoraria from Merck Sharp and Dohme, Pierre Fabre, Sunpharma and Sanofi Genzyme, and travel support from Bristol Myers Squibb, and Pierre Fabre. CL declares speakers and advisory board honoraria and travel support from Bristol Myers Squibb, Merck Sharp and Dohme, Merck Serono, Novartis, Roche, Amgen, Pierre Fabre, and Sun Pharma. CP received speaker or consultant honoraria and travel support from Novartis, Bristol Myers Squibb, Roche, Merck Serono, Merck Sharp and Dohme, Celgene, AbbVie, and LEO. LH received grants from Novartis, and has received speaker or consultant fees personal fees from Amgen, Bristol Myers Squibb, Merck Sharp and Dohme, Roche, Curevac, Pierre Fabre, Roche, Novartis, and Sanofi. MK has received grants from Bristol Myers Squibb, Merck Sharp and Dohme, Leo, Novartis, and Roche. DG declares speakers and advisory honoraria as well as travel support from Bristol Myers Squibb, Novartis, Pierre Fabre, Sanofi Genzyme, Amgen, Galderma, Janssen, and Roche. DS declares advisory board and speakers honoraria from Roche, Novartis, Bristol-Myers-Squibb, MSD, Merck-Serono, Sanofi, Nektar, Amgen, Hexal, InFlaRx, Array, Pierre Fabre, Immunocore, Philogen Sun Pharma, Regeneron, and Ultimovacs, as well as grant and travel support from Roche, Novartis, Bristol-Myers-Squibb, MSD, Merck-Serono, and Sanofi. SU declares research support from Bristol Myers Squibb, and Merck Serono, speakers and advisory board honoraria from Bristol Myers Squibb, Merck Sharp and Dohme, Merck Serono, Novartis and Roche, and travel support from Bristol Myers Squibb, Merck Sharp, and Dohme. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross MI, et al. . For Members of the American Joint Committee on Cancer Melanoma Expert Panel and the International Melanoma Database and Discovery Platform. Melanoma Staging: Evidence-based Changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J Clin (2017) 67(6):472–92. 10.3322/caac.21409 - DOI - PMC - PubMed
-
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. . New Guidelines to Evaluate the Response to Treatment in Solid Tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst (2000) 92(3):205–16. 10.1093/jnci/92.3.205 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

