The Road Less Traveled? Unconventional Protein Secretion at Parasite-Host Interfaces
- PMID: 34109175
- PMCID: PMC8182054
- DOI: 10.3389/fcell.2021.662711
The Road Less Traveled? Unconventional Protein Secretion at Parasite-Host Interfaces
Abstract
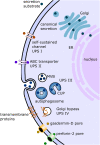
Protein secretion in eukaryotic cells is a well-studied process, which has been known for decades and is dealt with by any standard cell biology textbook. However, over the past 20 years, several studies led to the realization that protein secretion as a process might not be as uniform among different cargos as once thought. While in classic canonical secretion proteins carry a signal sequence, the secretory or surface proteome of several organisms demonstrated a lack of such signals in several secreted proteins. Other proteins were found to indeed carry a leader sequence, but simply circumvent the Golgi apparatus, which in canonical secretion is generally responsible for the modification and sorting of secretory proteins after their passage through the endoplasmic reticulum (ER). These alternative mechanisms of protein translocation to, or across, the plasma membrane were collectively termed "unconventional protein secretion" (UPS). To date, many research groups have studied UPS in their respective model organism of choice, with surprising reports on the proportion of unconventionally secreted proteins and their crucial roles for the cell and survival of the organism. Involved in processes such as immune responses and cell proliferation, and including far more different cargo proteins in different organisms than anyone had expected, unconventional secretion does not seem so unconventional after all. Alongside mammalian cells, much work on this topic has been done on protist parasites, including genera Leishmania, Trypanosoma, Plasmodium, Trichomonas, Giardia, and Entamoeba. Studies on protein secretion have mainly focused on parasite-derived virulence factors as a main source of pathogenicity for hosts. Given their need to secrete a variety of substrates, which may not be compatible with canonical secretion pathways, the study of mechanisms for alternative secretion pathways is particularly interesting in protist parasites. In this review, we provide an overview on the current status of knowledge on UPS in parasitic protists preceded by a brief overview of UPS in the mammalian cell model with a focus on IL-1β and FGF-2 as paradigmatic UPS substrates.
Keywords: glycolysis; host–pathogen interface; moonlighting; protist parasites; unconventional secretion.
Copyright © 2021 Balmer and Faso.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

